
Helping you stay on top of the latest developments in the medical device industry.
Stay Up-to-Date with our Monthly MedTechFuture Newsletter

Everything you Need to Know About the European Assisted Reproduction Technology Market
The European assisted reproduction technology (ART) market is evolving rapidly, driven by advancements in medical technology, varied national healthcare policies, and a growing demand for fertilit

Top Procedure Performed Within the US Urological Devices Market in 2023
Throughout 2023, the MedTech space has seen significant activity in the realm of urological procedures, driven predominantly by an aging population vulnerable to various urological disorders. Amon

Hologic, Inc. Expands Women’s Health Portfolio with Strategic Acquisition of Endomagnetics Ltd
Hologic, Inc., a leading innovator in women's health, recently announced its strategic acquisition of Endomagnetics Ltd (Endomag®), a key player in breast cancer surgery technologies. This acquis

What Impact Have Recent Mergers, Acquisitions, and Technological Advances had on the Surgical Display and PACS Monitor Market Globally?
In the landscape of medical technology, advancements in surgical displays and PACS are revolutionizing the way healthcare professionals diagnose and treat patients. From groundbreaking partnership

Top Developments in the Global Anesthesia, Respiratory, and Sleep Therapy Market: 2023 and 2024
The landscape of the global anesthesia, respiratory, and sleep therapy market has been vibrant with activity over the past two years, marking significant shifts, technological advancements, and st

Carl Zeiss Meditec AG Finalizes Takeover of Dutch Ophthalmic Research Center (D.O.R.C.)
Carl Zeiss Meditec AG recently announced the successful completion of its acquisition of the Dutch Ophthalmic Research Center (D.O.R.C.), a pivotal move that marks a significant milestone in the o

Haemonetics’ Strategic Acquisition of Attune Medical and the Future of Esophageal Cooling Technology
Haemonetics Corporation, a leading global medical technology firm, has announced its plans to acquire Attune Medical, a Chicago-based company known for its groundbreaking ensoETM® esophageal coolin

Highlights from AAOS 2024: CytexOrtho, Triathlon Hinge, CartiHeal Agili-C, and more
The 2024 American Academy of Orthopaedic Surgeons (AAOS) meeting held in San Francisco was undeniably a standout event, particularly given the heightened anticipation following the prolonged impac

How Can Data Be Used to Assist Mergers and Acquisitions?
Mergers and acquisitions are major decisions in an organization's life. M&As can sometimes be a case of knowing when you know. These decisions, however, usually require data to support them. U

Virtual and Augmented Reality Innovations Take Center Stage at AAOS 2024
AAOS 2024 marked an exceptional event, boasting a plethora of innovative devices, debut exhibitors, and cutting-edge technology. Among the array of advancements showcased during the event, virtual

Boston Scientific to Acquire Axonics in $3.7B Deal: Transforming the Landscape of Urology and Neuromodulation
Boston Scientific Corporation (NYSE: BSX) has disclosed its definitive agreement to purchase Axonics, Inc. (Nasdaq: AXNX), a publicly traded medical technology firm primarily dedicated to advancin

Revolutionizing Glaucoma Treatment: iDose TR Receives FDA Approval for Groundbreaking Micro-Invasive Therapy
In a groundbreaking development for glaucoma patients, Glaukos Corporation, a leading ophthalmic medical technology and pharmaceutical company, has received approval from the U.S. Food and Drug Ad

The Technological Revolution in the US Patient Monitoring Equipment Market
In the United States, there is a profound evolution underway in the realm of Patient Monitoring Equipment, driven by remarkable technological progress. The spectrum of advancements spans from cent

How Many Laparoscopic Procedures Were Performed in 2023?
Over the past year, laparoscopy has reshaped the medical landscape, promising swifter patient recovery, shorter hospital stays, and a notable reduction in post-operative complications. Laparoscopy

Top Performers in the U.S. Central Station Monitors Market – Insights from iData’s MedSKU
CARESCAPE Central Station, retrieved from: https://www.gehealthcare.com/products/patient-monitoring/patient-monitors/carescape-central-station In iData's comprehensive analysis of the U.S. Patient

Top 10 Wound Care Companies in the United States
The total U.S. market for wound care is growing at a rate of 4.6%, which will take the 2018 market value of $10 billion up to $15 billion by 2025. As a result of the large market increase, we decided

Post-COVID-19 Growth Trajectory of the European Anesthesia, Respiratory, and Sleep Therapy Market
The year 2020 brought unprecedented challenges to various sectors, and the healthcare industry was no exception. In a comprehensive market analysis, the impact of COVID-19 on the European anesthes

Merck to Acquire Caraway Therapeutics in $610 Million Deal
Merck (NYSE: MRK), also known as MSD outside of the United States and Canada, and Caraway Therapeutics, Inc. have entered into a definitive agreement for Merck to acquire Caraway Therapeutics in a

The 4K Technology Surge in the Global Video and Integrated Operating Room Market
Retrieved from: https://pro.sony/en_CA/healthcare/imaging-innovations-insights/operating-room-4k-visualisationIn the ever-evolving field of modern medicine, technological advancements consiste

Holiday Specials with iData
This December, revel in the festivities with iData Research's exclusive Holiday Promotions , featuring special offers valid until January 1st across our comprehensive range of Global MedCore repor

The Sound of Progress: Key Developments in the Global Hearing Device Market
The world of audiology and hearing devices has witnessed significant strides in recent times, with several remarkable developments that promise to enhance the quality of life for individuals with

Understanding the Benefits of Ophthalmic Device Consolidation
The field of ophthalmology is constantly evolving, and so too are the devices and technologies used by ophthalmologists to diagnose and treat various eye conditions. One prominent trend that has b

Mastering the Healthcare Market: Why GPOs Need Market Research for Success
In the fast-paced and fiercely competitive healthcare sector, maintaining a competitive edge is essential for achieving success. One of the key tools that organizations, especially Group Purchasin

Top 6 Market Developments in the U.S. Video and Integrated Operating Room Equipment Market
The landscape of surgical technology is ever-evolving, with innovations continuously reshaping the way procedures are conducted. Throughout 2022 and 2023, the U.S. video and integrated operating r

US Medical Imaging 2023 Industry Updates: Advancements Reshaping the Market
The year 2023 has proven to be a pivotal time for the U.S. medical imaging industry in the United States, with several groundbreaking developments that promise to redefine the landscape of healthc

Global Neurological Devices Market: A Comprehensive Regional Analysis
In the dynamic realm of healthcare technology, neurological devices play a pivotal role, reshaping the landscape of patient care. Our exploration begins with North America, a beacon of innovation

Exploring the Leaders in Aesthetic Laser and Light Therapy: A 2022 Overview
In the fast-evolving landscape of aesthetic laser and light therapy, 2022 witnessed the dominance of key players shaping the industry. Among them, Cynosure, a Clayton, Dubilier & Rice-owned co

Carlyle Group Eyes a $7 Billion Medical Marvel: Medtronic’s Health Pivot
In a strategic move that could reshape the landscape of medical innovation, private equity powerhouse Carlyle Group Inc is reportedly in the final stages of exclusive negotiations to secure a majo

Key Players in the Global Gastrointestinal Endoscopic Device Market: A Deep Dive
Welcome to our in-depth exploration of the global gastrointestinal (GI) endoscopic device market, where we take you on a journey to uncover the key players shaping the landscape of this dynamic in

What are the Most Popular BPH Treatment Devices by Brand in the U.S.?
Boston Scientific's Rezum Product. Retrieved From: https://www.bostonscientific.com/en-US/products/lithotripsy/rezum-water-vapor-therapy.html iData Research recently completed a detailed

Global Infusion Therapy Market: 5 Pivotal Trends Making Waves
Infusion therapy devices refer to medical equipment utilized for delivering fluids or medications directly into patients' veins. This method is favoured when patients require a swifter alternative

The Future of Medtech: Exploring the Impact of Layoffs on Innovation and Advancements
In an age of rapid technological advancements, the medtech industry stands at the forefront of innovation. From artificial intelligence and robotics to telemedicine and wearable devices, the possi

The Evolution of Laparoscopic Surgery: Trends and Innovations in the European Market
In recent years, the field of surgery has undergone a remarkable transformation with the rise of minimally invasive techniques. Laparoscopic surgery, also known as keyhole surgery, has t
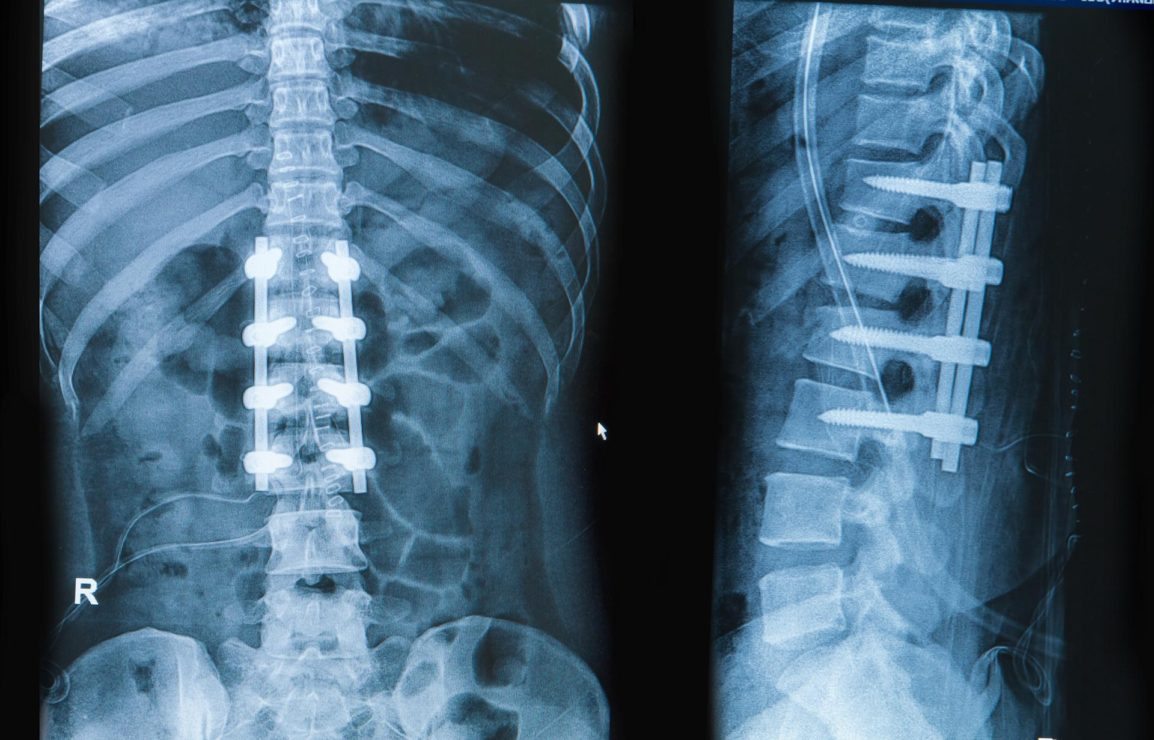
How Many Spinal Fusions are Performed Each Year in the United States?
Register to receive a free US Spinal Implants and VCF Market Report Suite synopsis and brochure The task of researching how many spinal fusions are performed each year is actually more complex than

Top Smith & Nephew Competitors in the United States
Register to receive a free synopsis and brochure. Browse the Report LibraryMore than 160 years ago, Smith & Nephew began its operations in the UK. Today, it is a global medical technology
Balancing Cost and Care: The Shift to Less Expensive Catheters in the U.S. Vascular Access Device Market
In the ever-evolving landscape of healthcare, innovations and advancements continue to shape the way we approach patient care. One such transformation is occurring in the U.S. Vascular Access Devi

Exploring the Dynamics of the U.S. Gynecological Devices Market
The U.S. gynecological devices market is expected reach a market value of $1.3 Billion by 2029 as technological advancements, shifting demographics, and changing healthcare practices shape its lan

Identifying the Driving Forces of the European Infusion Therapy Device Market
The European Infusion Therapy Device Market demonstrates resilience in the face of economic uncertainties across the continent. Despite an impending recession, the market continues to grow steadil
Investigating the Drivers of Sustained Growth in the U.S. Laparoscopy Device Market
The U.S. Laparoscopy Device Market is expected to reach a market value of at least $5.6 Billion by 2029. In 2022 alone, more than 2 million of these minimally invasive laparoscopic surgeries were

Everything You Need to Know About the European Transcatheter Embolization Market
The European Transcatheter Embolization Market is expected to see steady growth over the next 6 years. In 2022, the European Transcatheter Embolization Market was worth just under $290 Million. By

Charting the Path to Success: The Top 3 Hearing Aid Market Leaders
The global hearing aid market exhibited a valuation of $12 billion in 2021 and is projected to reach $19 billion by 2028. As the leading market segment in the broader hearing devices market, the h

Exploring the Video and Integrated Operating Room Market: A Look into the Future of Surgery
The integration of operating rooms and video endoscopy systems has brought about a revolutionary change in the surgical industry, significantly improving the efficiency and success rates of surgic

The World of Breast Implants: Segmentation and Product Concerns
Breast augmentation has been one of the most popular cosmetic surgeries in the world, with just under 300,000 procedures performed in the United States in 2020 alone. The global market f

Starkey’s Latest Innovative AI Hearing Aid Platform and Its Impact on the Industry
With the release of the Starkey Evolv AI, Starkey Hearing Technologies is setting a new precedent in the hearing device industry and illustrating the importance of technological innovation within th
Top 3 Market Competitors in the Global Botulinum Toxin A Market: Strategies and Positioning
The popularity of Botulinum Toxin A, also known as Botox®, has significantly increased, primarily due to societal norms and beauty standards promoted by social media. The market value of this tre

Exploring the Growth of the Neuromodulation Market: Key Drivers and Trends
Last year, more than 215,000 neuromodulation procedures were performed worldwide, with expectations that this number will climb to nearly 400,000 by 2029. Furthermore, it is expected that the mark

Exploring Empowerment: iData and Pebbles to Pearls Foundation Visit Barli Institute for Rural Development in India
iData staff (https://idataresearch.com/about-us/) along with Pebbles to Pearls Foundation (https://www.pebblestopearlsfoundation.ca), are currently co-visiting Barli Institute for Rural Development

From Catheters to Ports: A Deep Dive into the Latest Trends in Vascular Access Devices
Vascular access devices are becoming increasingly popular in the United States. However, during the COVID-19 pandemic, the quantity of units purchased decreased, and this trend continued into 2022

Olympus to Acquire Taewoong Medical: Strengthening its GI Endotherapy Portfolio for Improved Patient Outcomes
Olympus Corporation, a renowned global medtech company known for its innovative solutions that enhance people's lives, has announced a new acquisition. Taewoong Medical Co., Ltd, a leading Korean

Inside the U.S. ERCP Device Market: A Closer Look at Key Players
ERCP is a medical procedure that utilizes endoscopy and fluoroscopy to diagnose, treat, and examine conditions in the liver, gallbladder, bile duct, and pancreas. Typically, an ERCP involves a sur

Behind the Scenes: The Thriving Artificial Disc Market and the Underdog Fighting to Catch Up
The artificial disc market has been on a steady rise in recent years, and in 2021, it reached new heights with a double-digit growth rate. Despite the challenges posed by the COVID-19 pandemic, th
Navigating the Neonatal Ventilator Market: A Brand-Level Analysis into the Post-Pandemic Landscape
The demand for ventilators underwent a significant surge due to the onset of the COVID-19 pandemic. The global health crisis put a spotlight on the importance of ventilators and the critical role th

The Hidden Risks of Healthcare M&A: How Market Research Can Uncover Them
Mergers and acquisitions (M&A) can be a lucrative way for private equity (PE) firms to invest in the healthcare industry. However, there are also hidden risks associated with the healthcare se

Weekly Round-Up: Dexcom’s 20% 2023 Revenue Growth, GE Healthcare Buys IMACTIS, Rethinking 510(k)
Things seem to be happening all the time in the medical device industry. Whether it's M&As, product launches, FDA approvals, or recalls, there's never a dull moment. In light of the fact that ever

What Are the Factors Driving Growth in the U.S. Interventional Cardiology Market?
Register to receive a free Interventional Cardiology Devices Market Report Suite for U.S. 2023-2029 synopsis and brochure Coronary artery disease (CAD) is a multifaceted disease that demands variou

Top 5 Surprising Developments in the U.S. ENT Market
According to the latest research by iData Research, many segments of the U.S. ENT market are relatively commoditized. As companies compete to gain market share in these segments, innovation is lacking

Top M&A Deals in the Global Video Endoscopy Market
Based on a recent report published by iData Research on the global video endoscopy market, several mergers, acquisitions, and deals occurred within the past few years. Video endoscopy systems are

ICYMI: First-Ever Human Study for Stress Urinary Incontinence Was Initiated by UroMems
On November 29th, 2022 UroMems announced that they had successfully completed the first-in-human implant of the UroActive™ System to treat stress urinary incontinence. According to Hamid Lamraou

Smarter Operating Rooms Are Saving More Lives: The Impact of Hybrid Operating Rooms
Register to receive a free Global Video and Integrated Operating Room Equipment Market Report Suite synopsis According to a new series of reports by iData Research, the Global video and integrated op

Top 5 Surprising Developments in the Global Hearing Device Market
According to the latest research by iData Research, the global hearing device market is limited by lifestyle and aesthetic concerns. Despite the many new hearing aid styles and discreet designs, s

Top NuVasive Competitors in the U.S. Spine Markets
Register to receive a free Minimally Invasive Spinal Implants Market Report Suite for US synopsis and brochure NuVasive, Inc. is a global medical device company founded in 1999 and focused on devel
