Product Description
Overall, Japan’s spinal implants market was valued at $550 million in 2021. However, this is expected to increase over the forecast period at a CAGR of 1.4% to reach $605 million by 2028.
Currently, the top competitors within South Korea’s spinal implants market are Medtronic, DePuy Synthes, and Stryker. Medtronic was the leading competitor in the spinal implant market in Japan.
Throughout this medical market research, we analyzed over 10 spinal implant companies and used our comprehensive methodology to understand the market sizes, unit sales, company market shares, procedure volumes, and to create accurate forecasts.
MARKET DATA INCLUDED
- Unit Sales, Average Selling Prices, Market Size & Growth Trends
- Procedure Numbers
- COVID-19 Impact Analysis
- Market Drivers & Limiters
- Market Forecasts Until 2028, and Historical Data to 2018
- Recent Mergers & Acquisitions
- Company Profiles and Product Portfolios
- Leading Competitors
PROCEDURE NUMBERS
 Throughout this study on Japan’s spinal implants market, iData Research has performed an analysis on cervical fixation procedures, thoracolumbar fixation procedures, interbody fusion procedures, motion preservation procedures, and vertebral compression fracture procedures to increase the accuracy of our market sizing and provide you with the volume of procedures over a 10-year range.
Throughout this study on Japan’s spinal implants market, iData Research has performed an analysis on cervical fixation procedures, thoracolumbar fixation procedures, interbody fusion procedures, motion preservation procedures, and vertebral compression fracture procedures to increase the accuracy of our market sizing and provide you with the volume of procedures over a 10-year range.
JAPAN’S SPINAL IMPLANTS MARKET INSIGHTS
 One ongoing trend in the Japan market is favorable demographics. Both fusion and non-fusion procedures are typically performed in order to treat spinal complications that are much more common in elderly patients. As of 2020, approximately 28% of the Japanese population was over 65 years of age. By 2036, those over 65 will comprise over a third of the population. Growth of this population is expected to stimulate growth in both fusion and non-fusion spinal implant markets.
One ongoing trend in the Japan market is favorable demographics. Both fusion and non-fusion procedures are typically performed in order to treat spinal complications that are much more common in elderly patients. As of 2020, approximately 28% of the Japanese population was over 65 years of age. By 2036, those over 65 will comprise over a third of the population. Growth of this population is expected to stimulate growth in both fusion and non-fusion spinal implant markets.
MARKET SEGMENTATION SUMMARY
- Procedure Numbers for Spinal Implants and VCF – Includes:
- Cervical Fixation Procedures, Thoracolumbar Fixation Procedures, Interbody Device Procedures, Motion Preservation Procedures, Vertebral Compression Fracture Procedures, Spinal Electrical Stimulation Device Procedures
- Cervical Fixation Market – Further Segmented Into:
- Anterior Cervical Fixation Market, Posterior Cervical Fixation Market
- Thoracolumbar Fixation Market – Further Segmented Into:
- Degenerative Thoracolumbar Fixation Market, Deformity Correction Thoracolumbar Fixation Market, Trauma/Tumor Thoracolumbar Fixation Market
- Interbody Device Market – Further Segmented Into:
- ALIF Market, PLIF Market, TLIF Market, and CIF Market, VBR/Corpectomy Market, Expandable Interbody Device Market, Standalone Interbody Device Market
- Motion Preservation Market – Further Segmented Into:
- Artificial Disc Market, Dynamic Stabilization Market
- Vertebral Compression Market – Further Segmented Into:
- Vertebral Compression Fracture Market, Vertebroplasty Market, Percutaneous Vertebral Augmentation Market
- Electrical Stimulation Device Market – Further Segmented Into:
- Cervical Electrical Stimulation Devices and Lumbar Electrical Stimulation Devices
- Spinal Surgery Instrumentation Market – Includes:
- Data aggregated for tools not otherwise mentioned in other segments and procedures – for example, scalpels, catheters, rongeurs, clamps, forceps, retractors, and cannula, in addition to other products.
- Neuromonitoring Market – Further Segmented Into:
- Neuromonitoring Device Market, Neuromonitoring System Market, Neuromonitoring Accessory Market
JAPAN SPINAL IMPLANTS MARKET SHARE INSIGHTS
The spinal implants market in Japan was controlled byMedtronic, DePuySynthes, and Stryker. Medtronic was the leading competitor in every major segment except for vertebral compression fracture (VCF). The company offers an extensive portfolio in all segments, which has allowed for the company’s continued success within the market. DePuy Synthes was the second-leading competitor in the spinal implant and VCF market. It was the second-leading competitor in all major market segments. The company’s largest market share was in the thoracolumbar fixation market segment.
Medtronic was the leading competitor in every major segment except for vertebral compression fracture (VCF). The company offers an extensive portfolio in all segments, which has allowed for the company’s continued success within the market. DePuy Synthes was the second-leading competitor in the spinal implant and VCF market. It was the second-leading competitor in all major market segments. The company’s largest market share was in the thoracolumbar fixation market segment.
RESEARCH SCOPE SUMMARY
| Report Attribute | Details |
|---|
| Region | Japan |
| Base Year | 2021 |
| Forecast | 2022-2028 |
| Historical Data | 2018-2021 |
| Quantitative Coverage | Procedure Numbers, Market Size, Market Shares, Market Forecasts, Market Growth Rates, Units Sold, and Average Selling Prices. |
| Qualitative Coverage | COVID-19 Impact, Market Growth Trends, Market Limiters, Competitive Analysis & SWOT for Top Competitors, Mergers & Acquisitions, Company Profiles, Product Portfolios, Disease Overviews. |
| Data Sources | Primary Interviews with Industry Leaders, Government Physician Data, Regulatory Data, Hospital Private Data, Import & Export Data, iData Research Internal Database. |
FREE Sample Report
TABLE OF CONTENTS
TABLE OF CONTENTS I
LIST OF FIGURES XI
LIST OF CHARTS XVIII
EXECUTIVE SUMMARY 1
JAPAN SPINAL IMPLANT AND VCF MARKET OVERVIEW 1
COMPETITIVE ANALYSIS 3
MARKET TRENDS 5
MARKET DEVELOPMENTS 7
PROCEDURE NUMBERS 10
MARKETS INCLUDED 11
KEY REPORT UPDATES 13
VERSION HISTORY 13
RESEARCH METHODOLOGY 14
Step 1: Project Initiation & Team Selection 14
Step 2: Prepare Data Systems and Perform Secondary Research 17
Step 3: Preparation for Interviews & Questionnaire Design 19
Step 4: Performing Primary Research 20
Step 5: Research Analysis: Establishing Baseline Estimates 22
Step 6: Market Forecast and Analysis 23
Step 7: Identify Strategic Opportunities 25
Step 8: Final Review and Market Release 26
Step 9: Customer Feedback and Market Monitoring 27
IMPACT OF COVID-19 ON THE JAPAN SPINAL IMPLANT AND VCF MARKET 28
2.1 INTRODUCTION 28
2.2 ANALYSIS BY MARKET SEGMENT 30
2.2.1 Worst Case Scenario 30
2.2.2 Base Case Scenario 31
2.2.3 Best Case Scenario 32
DISEASE OVERVIEW 34
3.1 INTRODUCTION 34
3.1.1 Degenerative Disc Disease 34
3.1.1.1 Disc Herniation 34
3.1.1.2 Stenosis 35
3.1.1.3 Spondyloisthesis 35
3.1.1.4 Arthritis 36
3.1.2 Spinal Deformities 36
3.1.2.1 Scoliosis 36
3.1.2.2 Kyphosis and Lordosis 37
3.1.3 Trauma and Tumor 38
3.1.4 Vertebral Compression Fractures 38
3.1.4.1 Osteoporosis 38
3.1.4.2 Vertebral Compression Fractures 39
PRODUCT ASSESSMENT 40
4.1 INTRODUCTION 40
4.2 PRODUCT PORTFOLIOS 40
4.2.1 Cervical Fixation 40
4.2.1.1 Anterior Cervical Fixation 41
4.2.1.1.1 Constrained 41
4.2.1.1.2 Semi-Constrained 41
4.2.1.1.3 Dynamic 41
4.2.1.1.4 Bioresorbable 42
4.2.1.2 Posterior Cervical Fixation 42
4.2.2 Facet Fixation 45
4.2.3 Interbody Devices 47
4.2.3.1 Approaches 47
4.2.3.1.1 ALIF 47
4.2.3.1.2 PLIF 48
4.2.3.1.3 TLIF 48
4.2.3.1.4 Minimally Invasive PLIF 48
4.2.3.1.5 Minimally Invasive TLIF 49
4.2.3.1.6 LLIF 49
4.2.3.1.7 OLIF 49
4.2.3.1.8 Cervical 49
4.2.3.1.9 VBR/Corpectomy 50
4.2.3.2 Materials 50
4.2.3.2.1 Metal 50
4.2.3.2.2 PEEK 50
4.2.3.2.3 Machined Bone Allograft 50
4.2.4 MIS Sacroiliac Joint Fusion 54
4.2.5 Motion Preservation Devices 56
4.2.5.1 Artificial Discs 56
4.2.5.2 Dynamic Stabilization 56
4.2.5.2.1 Posterior Dynamic Stabilization 56
4.2.5.2.2 Interspinous Process Decompression 56
4.2.5.2.3 Nucleus Replacement 57
4.2.5.2.4 Facet Arthroplasty 57
4.2.6 Spinous Process Fixation 60
4.2.7 Spinal Electrical Stimulation Devices 62
4.2.8 Spine Endoscopes 64
4.2.9 Thoracolumbar Fixation 66
4.2.9.1 Traditional Systems 66
4.2.9.2 Minimally Invasive Systems 66
4.2.10 Vertebral Compression Fracture Devices 70
4.2.10.1 Percutaneous Vertebroplasty 70
4.2.10.2 Percutaneous Vertebral Augmentation Including Kyphoplasty 71
4.3 REGULATORY ISSUES AND RECALLS 73
4.3.1 Medtronic 73
4.3.2 VGI Medical 74
4.3.3 Zimmer Biomet 75
4.4 CLINICAL TRIALS 78
4.4.1 Cervical Fixation 78
4.4.1.1 Other Companies 78
4.4.2 Interbody Devices 79
4.4.2.1 Other Companies 79
4.4.3 Facet Fixation 81
4.4.3.1 Medtronic 81
4.4.3.2 Other Companies 82
4.4.4 Percutaneous Vertebroplasty 82
4.4.4.1 Other Companies 82
JAPAN SPINAL IMPLANT AND VCF MARKET OVERVIEW 84
5.1 INTRODUCTION 84
5.1.1 Spinal Fusion 84
5.1.2 Traditional Interbody Fusion 85
5.1.2.1 ALIF 85
5.1.2.2 PLIF 86
5.1.2.3 TLIF 86
5.1.2.4 CIF 86
5.1.3 Motion Preservation 86
5.1.3.1 Artificial Discs 87
5.1.4 Vertebral Compression Fracture (VCF) 87
5.1.5 Electrical Stimulation 87
5.1.6 Spinal Surgery Instrumentation 88
5.2 CURRENCY EXCHANGE RATE 89
5.3 MARKET OVERVIEW & TREND ANALYSIS 90
5.4 DRIVERS AND LIMITERS 94
5.4.1 Market Drivers 94
5.4.2 Market Limiters 94
5.5 COMPETITIVE MARKET SHARE ANALYSIS 96
5.6 MERGERS AND ACQUISITIONS 101
5.7 COMPANY PROFILES 105
5.7.1 DePuy Synthes 105
5.7.2 Globus Medical 106
5.7.3 Joimax 108
5.7.4 Medtronic 109
5.7.5 NuVasive 110
5.7.6 SI-BONE 111
5.7.7 Stryker 112
5.7.8 VGI Medical 113
5.7.9 Zimmer Biomet 114
5.8 SWOT ANALYSIS 116
5.8.1 DePuy Synthes 117
5.8.2 Globus Medical 118
5.8.3 Joimax 119
5.8.4 Medtronic 120
5.8.5 NuVasive 121
5.8.6 SI-BONE 123
5.8.7 Stryker 124
5.8.8 VGI Medical 126
5.8.9 Zimmer Biomet 127
PROCEDURE NUMBERS 128
6.1 INTRODUCTION 128
6.2 PROCEDURES 129
6.2.1 Cervical Fixation Procedures 132
6.2.1.1 Anterior Cervical Fixation Procedures 134
6.2.1.2 Posterior Cervical Fixation Procedures 136
6.2.2 Thoracolumbar Fixation Procedures 138
6.2.2.1 Degenerative Thoracolumbar Fixation Procedures 140
6.2.2.1.1 Anterior Degenerative Thoracolumbar Fixation Procedures 143
6.2.2.1.2 Posterior Degenerative Thoracolumbar Fixation Procedures 144
6.2.2.2 Deformity Correction Thoracolumbar Fixation Procedures 146
6.2.2.3 Trauma/Tumor Thoracolumbar Fixation Procedures 147
6.2.3 Interbody Device Procedures 148
6.2.3.1 ALIF Procedures 151
6.2.3.2 PLIF Procedures 152
6.2.3.3 TLIF Procedures 153
6.2.3.4 CIF Procedures 154
6.2.3.5 VBR/Corpectomy Procedures 155
6.2.3.6 Expandable Interbody Procedures 156
6.2.3.7 Standalone Interbody Procedures 157
6.2.4 Vertebral Compression Fracture Procedures 158
6.2.4.1 Vertebroplasty Procedures 160
6.2.4.2 Percutaneous Vertebral Augmentation Procedures 161
CERVICAL FIXATION MARKET 162
7.1 INTRODUCTION 162
7.1.1 Anterior Cervical Fixation 163
7.1.1.1 Constrained 163
7.1.1.2 Semi-Constrained 163
7.1.1.3 Dynamic 163
7.1.1.4 Bioresorbable 163
7.1.2 Posterior Cervical Fixation 164
7.2 MARKET OVERVIEW 165
7.3 MARKET ANALYSIS AND FORECAST 168
7.3.1 Anterior Cervical Fixation Market 168
7.3.1.1 Constrained Anterior Cervical Fixation Market 172
7.3.1.2 Semi-Constrained Anterior Cervical Fixation Market 173
7.3.1.3 Dynamic Anterior Cervical Fixation Market 174
7.3.1.4 Bioresorbable Anterior Cervical Fixation Market 175
7.3.2 Posterior Cervical Fixation Market 177
7.3.2.1 Cable/Wiring Posterior Cervical Fixation Market 180
7.3.2.2 Screw/Rod Posterior Cervical Fixation Market 181
7.3.2.3 Occipitocervical Posterior Cervical Fixation Market 183
7.4 DRIVERS AND LIMITERS 184
7.4.1 Market Drivers 184
7.4.2 Market Limiters 185
7.5 COMPETITIVE MARKET SHARE ANALYSIS 186
THORACOLUMBAR FIXATION MARKET 189
8.1 INTRODUCTION 189
8.1.1 Degenerative Thoracolumbar Fixation 190
8.1.2 Thoracolumbar Deformity Correction 190
8.1.3 Trauma/Tumor Thoracolumbar Fixation 190
8.2 MARKET OVERVIEW 191
8.3 MARKET ANALYSIS AND FORECAST 195
8.3.1 Degenerative Thoracolumbar Fixation Market 195
8.3.1.1 Degenerative Thoracolumbar Fixation Market by Approach 197
8.3.1.1.1 Anterior Plating Degenerative Thoracolumbar Fixation Market 199
8.3.1.1.2 Posterior Plating Degenerative Thoracolumbar Fixation Market 200
8.3.1.2 Degenerative Thoracolumbar Fixation Market by Levels Treated 201
8.3.1.2.1 Single-Level Degenerative Thoracolumbar Fixation Market 203
8.3.1.2.2 Two-Level Degenerative Thoracolumbar Fixation Market 204
8.3.1.2.3 Multi-Level Degenerative Thoracolumbar Fixation Market 205
8.3.2 Deformity Correction Thoracolumbar Fixation Market 206
8.3.3 Trauma/Tumor Thoracolumbar Fixation Market 208
8.4 DRIVERS AND LIMITERS 209
8.4.1 Market Drivers 209
8.4.2 Market Limiters 210
8.5 COMPETITIVE MARKET SHARE ANALYSIS 211
INTERBODY DEVICE MARKET 215
9.1 INTRODUCTION 215
9.2 MARKET OVERVIEW 219
9.3 MARKET ANALYSIS AND FORECAST 223
9.3.1 Interbody Device Market 223
9.3.2 ALIF Market 225
9.3.2.1 Metal ALIF Market 228
9.3.2.2 PEEK ALIF Market 229
9.3.2.3 Machined Bone ALIF Market 230
9.3.3 PLIF Market 231
9.3.3.1 Metal PLIF Market 234
9.3.3.2 PEEK PLIF Market 235
9.3.3.3 Machined Bone PLIF Market 236
9.3.4 TLIF Market 237
9.3.4.1 Metal TLIF Market 240
9.3.4.2 PEEK TLIF Market 241
9.3.4.3 Machined Bone TLIF Market 242
9.3.5 CIF Market 243
9.3.5.1 Metal CIF Market 246
9.3.5.2 PEEK CIF Market 247
9.3.5.3 Machined Bone CIF Market 248
9.3.6 VBR/Corpectomy Market 249
9.3.6.1 Metal VBR/Corpectomy Market 252
9.3.6.2 PEEK VBR/Corpectomy Market 253
9.3.7 Expandable Interbody Device Market 254
9.3.7.1 Metal Expandable Interbody Device Market 257
9.3.7.2 PEEK Expandable Interbody Device Market 258
9.3.8 Standalone Interbody Device Market 259
9.3.8.1 Cervical Standalone Interbody Device Market 262
9.3.8.2 Lumbar Standalone Interbody Device Market 263
9.4 DRIVERS AND LIMITERS 264
9.4.1 Market Drivers 264
9.4.2 Market Limiters 265
9.5 COMPETITIVE MARKET SHARE ANALYSIS 267
VERTEBRAL COMPRESSION FRACTURE MARKET 270
10.1 INTRODUCTION 270
10.1.1 Percutaneous Vertebroplasty 270
10.1.2 Percutaneous Vertebral Augmentation including Kyphoplasty 271
10.2 MARKET OVERVIEW 273
10.3 MARKET ANALYSIS AND FORECAST 276
10.3.1 Vertebral Compression Fracture Market 276
10.3.2 Vertebroplasty Market 277
10.3.3 Percutaneous Vertebral Augmentation Market 278
10.4 DRIVERS AND LIMITERS 279
10.4.1 Market Drivers 279
10.4.2 Market Limiters 280
10.5 COMPETITIVE MARKET SHARE ANALYSIS 282
SPINAL SURGERY INSTRUMENTATION MARKET 285
11.1 INTRODUCTION 285
11.2 MARKET ANALYSIS AND FORECAST 286
11.3 DRIVERS AND LIMITERS 289
11.3.1 Market Drivers 289
11.3.2 Market Limiters 289
11.4 COMPETITIVE MARKET SHARE ANALYSIS 291
APPENDIX: ELECTRICAL STIMULATION MARKET 293
12.1 INTRODUCTION 293
12.2 MARKET OVERVIEW 295
12.3 MARKET ANALYSIS AND FORECAST 298
12.3.1 Electrical Stimulation Market 298
12.3.2 Cervical Electrical Stimulation Market 299
12.3.3 Lumbar Electrical Stimulation Market 301
12.4 DRIVERS AND LIMITERS 303
12.4.1 Market Drivers 303
12.4.2 Market Limiters 304
ABBREVIATIONS 305
For more information on companies included,
contact iDataLIST OF CHARTS
Chart 1 1: Spinal Implant and VCF Market by Segment, Japan, 2021 – 2028 2
Chart 1 2: Spinal Implant and VCF Market Overview, Japan, 2021 & 2028 2
Chart 2 1: Multi-Scenario Spinal Implant and VCF Market Forecast, Japan, 2018 – 2028 (US$M) 33
Chart 5 1: Spinal Implant and VCF Market by Segment, Japan, 2018 – 2028 93
Chart 5 2: Leading Competitors, Spinal Implant and VCF Market, Japan, 2021 100
Chart 6 1: Spinal Implant and VCF Procedures by Segment, Japan, 2018 – 2028 131
Chart 7 1: Cervical Fixation Market by Segment, Japan, 2018 – 2028 167
Chart 7 2: Leading Competitors, Cervical Fixation Market, Japan, 2021 188
Chart 8 1: Thoracolumbar Fixation Market by Segment, Japan, 2018 – 2028 194
Chart 8 2: Leading Competitors, Thoracolumbar Fixation Market, Japan, 2021 214
Chart 9 1: Interbody Device Market by Segment, Japan, 2018 – 2028 222
Chart 9 2: Leading Competitors, Interbody Device Market, Japan, 2021 269
Chart 10 1: Vertebral Compression Fracture Market by Segment, Japan, 2018 – 2028 275
Chart 10 2: Leading Competitors, Vertebral Compression Fracture Market, Japan, 2021 284
Chart 11 1: Spinal Surgery Instrumentation Market, Japan, 2018 – 2028 288
Chart 12 1: Spinal Electrical Stimulation Device Market by Segment, Japan, 2018 – 2028 297
LIST OF FIGURES
Figure 1 1: Spinal Implant and VCF Market Share Ranking by Segment, Japan, 2021 (1 of 2) 3
Figure 1 2: Spinal Implant and VCF Market Share Ranking by Segment, Japan, 2021 (2 of 2) 3
Figure 1 3: Companies Researched in this Report 4
Figure 1 4: Factors Impacting the Spinal Implant and VCF Market by Segment, Japan (1 of 2) 5
Figure 1 5: Factors Impacting the Spinal Implant and VCF Market by Segment, Japan (2 of 2) 6
Figure 1 6: Recent Events in the Spinal Implant and VCF Market, Japan, 2018 – 2021 (1 of 3) 7
Figure 1 7: Recent Events in the Spinal Implant and VCF Market, Japan, 2018 – 2021 (2 of 3) 8
Figure 1 8: Recent Events in the Spinal Implant and VCF Market, Japan, 2018 – 2021 (3 of 3) 9
Figure 1 9: Spinal Implant and VCF Procedures Covered (1 of 3) 10
Figure 1 10: Spinal Implant and VCF Markets Covered (1 of 3) 11
Figure 1 11: Spinal Implant and VCF Markets Covered (2 of 3) 12
Figure 1 12: Spinal Implant and VCF Markets Covered (3 of 3) 12
Figure 1 13: Key Report Updates 13
Figure 1 14: Version History 13
Figure 2 1: Spinal Implant and VCF Market by Segment, Worst Case Scenario, Japan, 2018 – 2028 (US$M) 30
Figure 2 2: Spinal Implant and VCF Market by Segment, Base Case Scenario, Japan, 2018 – 2028 (US$M) 31
Figure 2 3: Spinal Implant and VCF Market by Segment, Best Case Scenario, Japan, 2018 – 2028 (US$M) 32
Figure 4 1: Cervical Fixation Devices by Company (1 of 2) 43
Figure 4 2: Cervical Fixation Devices by Company (2 of 2) 44
Figure 4 3: Facet Fixation Devices by Company 46
Figure 4 4: Interbody Devices by Company (1 of 3) 51
Figure 4 5: Interbody Devices by Company (2 of 3) 52
Figure 4 6: Interbody Devices by Company (3 of 3) 53
Figure 4 7: MIS Sacroiliac Joint Devices by Company 55
Figure 4 8: Cervical Artificial Disc Devices by Company 58
Figure 4 9: Lumbar Artificial Disc Devices by Company 58
Figure 4 10: Dynamic Posterior Stabilization Devices by Company 59
Figure 4 11: Interspinous Process Decompression Devices by Company 59
Figure 4 12: Spinous Process Fixation Devices by Company 61
Figure 4 13: Spinal Electrical Stimulation Devices by Company 63
Figure 4 14: Spine Endoscopes by Company 65
Figure 4 15: Thoracolumbar Fixation Devices by Company (1 of 3) 67
Figure 4 16: Thoracolumbar Fixation Devices by Company (2 of 3) 68
Figure 4 17: Thoracolumbar Fixation Devices by Company (3 of 3) 69
Figure 4 18: Vertebral Compression Fracture Devices by Company 72
Figure 4 19: Class 2 Device Recall Medtronic Prestige LP(TM) Cervical Disc System with Streamlined Instruments, DRILL GUIDES 73
Figure 4 20: Class 2 Device Recall Medtronic ZEVO(TM) Anterior Cervical Plate System SLOT SCREW 73
Figure 4 21: Class 2 Device Recall Vectris Trial Screening Lead Kit for Spinal Cord Stimulation 73
Figure 4 22: Class 2 Device Recall Vectris Trial Screening Lead Kit for Spinal Cord Stimulation 74
Figure 4 23: Class 2 Device Recall Medtronic CD HORIZON Spinal System MULTIAXIAL SCREW FOR 5.5/6.0MM RODS 74
Figure 4 24: Class 2 Device Recall VerteLP Instrument Kit 74
Figure 4 25: Class 2 Device Recall The MobiC Cervical Disc Prosthesis (MobiC; MobiC 14mm Distraction Screw 75
Figure 4 26: Class 2 Device Recall Lineum OCT Spine System 75
Figure 4 27: Class 2 Device Recall ROSA 1.0.2 (Spine application) 75
Figure 4 28: Class 2 Device Recall Polaris 5.5 Spinal System and Polaris 5.5/Cypher Spinal System 76
Figure 4 29: Class 2 Device Recall Vital MIS Spinal Fixation system 76
Figure 4 30: Class 2 Device Recall Lineum OCT Spine System 76
Figure 4 31: Class 2 Device Recall Zimmer Biomet Vitality Spinal Fixation System 77
Figure 4 32: Class 1 Device Recall SpF PLUSMini (60 A/W) Implantable Spinal Fusion Stimulator 77
Figure 4 33: Comparison of Short Segment Mono-axial and Poly-axial Pedicle Screw Fixation for Thoracolumbar Fractures 78
Figure 4 34: Prospective Study of Fusion Rates Using Spira-C Device for Anterior Cervical Discectomy and Fusion Surgery (ACDF) 79
Figure 4 35: Bio2 Vitrium® Cervical Cage in Anterior Cervical Discectomy and Fusion 79
Figure 4 36: SPIRA™-A 3D and HCT/p DBM vs. Medtronic Divergent™-L/Perimeter™ and Recombinant BMP-2 80
Figure 4 37: Retro-prospective Clinical Study to Evaluate the Efficacy of the Stand-alone Cervical Interbody Cage C-CURVE (Peek and Titanium) Manufactured by MEDICREA® (C-CURVE) 80
Figure 4 38: Percutaneous Screw Fixation vs. Open Fixation in the Treatment of Thoracolumbar Fractures (PrecScrew) 81
Figure 4 39: Clinical Outcome of Implant Removal Versus Retention After Posterior Fixation of Thoracolumbar Fractures 82
Figure 4 40: Early Percutaneous Vertebroplasty Versus Standard Conservative Treatment in Thoracolumbar Vertebral Fractures (AGIL11) 82
Figure 4 41: Percutaneous Vertebroplasty Using Rotary Cutter for Bone Metastases 83
Figure 5 1: Currency Exchange Rate, 2021 89
Figure 5 2: Spinal Implant and VCF Market by Segment, Japan, 2018 – 2028 (US$M) 91
Figure 5 3: Spinal Implant and VCF Market by Segment, Japan, 2018 – 2028 (JP¥M) 92
Figure 5 4: Leading Competitors, Spinal Implant and VCF Market, Japan, 2021 99
Figure 5 5: SWOT Analysis, DePuy Synthes 117
Figure 5 6: SWOT Analysis, Globus Medical 118
Figure 5 7: SWOT Analysis, Joimax 119
Figure 5 8: SWOT Analysis, Medtronic 120
Figure 5 9: SWOT Analysis, NuVasive (1 of 2) 121
Figure 5 10: SWOT Analysis, NuVasive (2 of 2) 122
Figure 5 11: SWOT Analysis, SI-BONE 123
Figure 5 12: SWOT Analysis, Stryker (1 of 2) 124
Figure 5 13: SWOT Analysis, Stryker (2 of 2) 125
Figure 5 14: SWOT Analysis, VGI Medical 126
Figure 5 15: SWOT Analysis, Zimmer Biomet 127
Figure 6 1: Spinal Implant and VCF Procedures by Segment, Japan, 2018 – 2028 130
Figure 6 2: Cervical Fixation Procedures by Segment, Japan, 2018 – 2028 133
Figure 6 3: Total Levels Treated, Cervical Fixation, Japan, 2018 – 2028 133
Figure 6 4: Anterior Cervical Fixation Procedures by Segment, Japan, 2018 – 2028 134
Figure 6 5: Total Anterior Cervical Fixation Procedures, Japan, 2018 – 2028 135
Figure 6 6: Posterior Cervical Fixation Procedures by Segment, Japan, 2018 – 2028 137
Figure 6 7: Total Posterior Cervical Fixation Procedures, Japan, 2018 – 2028 137
Figure 6 8: Thoracolumbar Fixation Procedures by Segment, Japan, 2018 – 2028 138
Figure 6 9: Total Levels Treated, Cervical Fixation, Japan, 2018 – 2028 139
Figure 6 10: Degenerative Thoracolumbar Fixation Procedures by Segment, Japan, 2018 – 2028 141
Figure 6 11: Total Levels Treated, Degenerative Thoracolumbar Fixation, Japan, 2018 – 2028 141
Figure 6 12: Total Degenerative Thoracolumbar Fixation Procedures, Japan, 2018 – 2028 142
Figure 6 13: Anterior Degenerative Thoracolumbar Fixation Procedures, Japan, 2018 – 2028 143
Figure 6 14: Posterior Degenerative Thoracolumbar Fixation Procedures, Japan, 2018 – 2028 144
Figure 6 15: Degenerative Thoracolumbar Fixation Procedures by Levels Treated, Japan, 2018 – 2028 145
Figure 6 16: Deformity Correction Thoracolumbar Fixation Procedures, Japan, 2018 – 2028 146
Figure 6 17: Trauma/Tumor Thoracolumbar Fixation Procedures, Japan, 2018 – 2028 147
Figure 6 18: Total Interbody Device Procedures by Segment, Japan, 2018 – 2028 149
Figure 6 19: Total Levels Treated, Japan, 2018 – 2028 150
Figure 6 20: ALIF Procedures, Japan, 2018 – 2028 151
Figure 6 21: PLIF Procedures, Japan, 2018 – 2028 152
Figure 6 22: TLIF Procedures, Japan, 2018 – 2028 153
Figure 6 23: CIF Procedures, Japan, 2018 – 2028 154
Figure 6 24: VBR/Corpectomy Procedures, Japan, 2018 – 2028 155
Figure 6 25: Expandable Interbody Procedures, Japan, 2018 – 2028 156
Figure 6 26: Standalone Interbody Procedures, Japan, 2018 – 2028 157
Figure 6 27: Vertebral Compression Fracture Procedures by Segment, Japan, 2018 – 2028 158
Figure 6 28: Total Levels Treated, Vertebral Compression Fracture, Japan, 2018 – 2028 159
Figure 6 29: Vertebroplasty Procedures, Japan, 2018 – 2028 160
Figure 6 30: Percutaneous Vertebral Augmentation Procedures, Japan, 2018 – 2028 161
Figure 7 1: Cervical Fixation Market by Segment, Japan, 2018 – 2028 (US$M) 166
Figure 7 2: Cervical Fixation Market by Segment, Japan, 2018 – 2028 (JP¥M) 166
Figure 7 3: Anterior Cervical Fixation Market by Segment, Japan, 2018 – 2028 (US$M) 169
Figure 7 4: Anterior Cervical Fixation Market by Segment, Japan, 2018 – 2028 (JP¥M) 170
Figure 7 5: Total Anterior Cervical Fixation Market, Japan, 2018 – 2028 171
Figure 7 6: Constrained Anterior Cervical Fixation Market, Japan, 2018 – 2028 172
Figure 7 7: Semi-Constrained Anterior Cervical Fixation Market, Japan, 2018 – 2028 173
Figure 7 8: Dynamic Anterior Cervical Fixation Market, Japan, 2018 – 2028 174
Figure 7 9: Bioresorbable Anterior Cervical Fixation Market, Japan, 2018 – 2028 176
Figure 7 10: Posterior Cervical Fixation Market by Segment, Japan, 2018 – 2028 (US$M) 178
Figure 7 11: Posterior Cervical Fixation Market by Segment, Japan, 2018 – 2028 (JP¥M) 178
Figure 7 12: Total Posterior Cervical Fixation Market, Japan, 2018 – 2028 179
Figure 7 13: Cable/Wiring Posterior Cervical Fixation Market, Japan, 2018 – 2028 180
Figure 7 14: Screw/Rod Posterior Cervical Fixation Market, Japan, 2018 – 2028 182
Figure 7 15: Occipitocervical Posterior Cervical Fixation Market, Japan, 2018 – 2028 183
Figure 7 16: Leading Competitors, Cervical Fixation Market, Japan, 2021 187
Figure 8 1: Thoracolumbar Fixation Market by Segment, Japan, 2018 – 2028 (US$M) 192
Figure 8 2: Thoracolumbar Fixation Market by Segment, Japan, 2018 – 2028 (JP¥M) 193
Figure 8 3: Total Degenerative Thoracolumbar Fixation Market, Japan, 2018 – 2028 196
Figure 8 4: Degenerative Thoracolumbar Fixation Market by Approach, Japan, 2018 – 2028 (US$M) 197
Figure 8 5: Degenerative Thoracolumbar Fixation Market by Approach, Japan, 2018 – 2028 (JP¥M) 198
Figure 8 6: Anterior Plating Degenerative Thoracolumbar Fixation Market, Japan, 2018 – 2028 199
Figure 8 7: Posterior Plating Degenerative Thoracolumbar Fixation Market, Japan, 2018 – 2028 200
Figure 8 8: Degenerative Thoracolumbar Fixation Market by Levels Treated, Japan, 2018 – 2028 (US$M) 201
Figure 8 9: Degenerative Thoracolumbar Fixation Market by Levels Treated, Japan, 2018 – 2028 (JP¥M) 202
Figure 8 10: Single-Level Degenerative Thoracolumbar Fixation Market, Japan, 2018 – 2028 203
Figure 8 11: Two-Level Degenerative Thoracolumbar Fixation Market, Japan, 2018 – 2028 204
Figure 8 12: Multi-Level Degenerative Thoracolumbar Fixation Market, Japan, 2018 – 2028 205
Figure 8 13: Deformity Correction Thoracolumbar Fixation Market, Japan, 2018 – 2028 207
Figure 8 14: Trauma/Tumor Thoracolumbar Fixation Market, Japan, 2018 – 2028 208
Figure 8 15: Leading Competitors, Thoracolumbar Fixation Market, Japan, 2021 213
Figure 9 1: Interbody Device Market by Segment, Japan, 2018 – 2028 (US$M) 220
Figure 9 2: Interbody Device Market by Segment, Japan, 2018 – 2028 (JP¥M) 221
Figure 9 3: Interbody Device Market, Japan, 2018 – 2028 224
Figure 9 4: ALIF Market by Segment, Japan, 2018 – 2028 (US$M) 226
Figure 9 5: ALIF Market by Segment, Japan, 2018 – 2028 (JP¥M) 226
Figure 9 6: Total ALIF Market, Japan, 2018 – 2028 227
Figure 9 7: Metal ALIF Market, Japan, 2018 – 2028 228
Figure 9 8: PEEK ALIF Market, Japan, 2018 – 2028 229
Figure 9 9: Machined Bone ALIF Market, Japan, 2018 – 2028 230
Figure 9 10: PLIF Market by Segment, Japan, 2018 – 2028 (US$M) 232
Figure 9 11: PLIF Market by Segment, Japan, 2018 – 2028 (JP¥M) 232
Figure 9 12: Total PLIF Market, Japan, 2018 – 2028 233
Figure 9 13: Metal PLIF Market, Japan, 2018 – 2028 234
Figure 9 14: PEEK PLIF Market, Japan, 2018 – 2028 235
Figure 9 15: Machined Bone PLIF Market, Japan, 2018 – 2028 236
Figure 9 16: TLIF Market by Segment, Japan, 2018 – 2028 (US$M) 238
Figure 9 17: TLIF Market by Segment, Japan, 2018 – 2028 (JP¥M) 238
Figure 9 18: Total TLIF Market, Japan, 2018 – 2028 239
Figure 9 19: Metal TLIF Market, Japan, 2018 – 2028 240
Figure 9 20: PEEK TLIF Market, Japan, 2018 – 2028 241
Figure 9 21: Machined Bone TLIF Market, Japan, 2018 – 2028 242
Figure 9 22: CIF Market by Segment, Japan, 2018 – 2028 (US$M) 244
Figure 9 23: CIF Market by Segment, Japan, 2018 – 2028 (JP¥M) 244
Figure 9 24: Total CIF Market, Japan, 2018 – 2028 245
Figure 9 25: Metal CIF Market, Japan, 2018 – 2028 246
Figure 9 26: PEEK CIF Market, Japan, 2018 – 2028 247
Figure 9 27: Machined Bone CIF Market, Japan, 2018 – 2028 248
Figure 9 28: VBR/Corpectomy Market by Segment, Japan, 2018 – 2028 (US$M) 250
Figure 9 29: VBR/Corpectomy Market by Segment, Japan, 2018 – 2028 (JP¥M) 250
Figure 9 30: Total VBR/Corpectomy Market, Japan, 2018 – 2028 251
Figure 9 31: Metal VBR/Corpectomy Market, Japan, 2018 – 2028 252
Figure 9 32: PEEK VBR/Corpectomy Market, Japan, 2018 – 2028 253
Figure 9 33: Expandable Interbody Device Market by Segment, Japan, 2018 – 2028 (US$M) 255
Figure 9 34: Expandable Interbody Device Market by Segment, Japan, 2018 – 2028 (JP¥M) 255
Figure 9 35: Total Expandable Interbody Device Market, Japan, 2018 – 2028 256
Figure 9 36: Metal Expandable Interbody Device Market, Japan, 2018 – 2028 257
Figure 9 37: PEEK Expandable Interbody Device Market, Japan, 2018 – 2028 258
Figure 9 38: Standalone Interbody Device Market by Segment, Japan, 2018 – 2028 (US$M) 260
Figure 9 39: Standalone Interbody Device Market by Segment, Japan, 2018 – 2028 (JP¥M) 260
Figure 9 40: Total Standalone Interbody Device Market, Japan, 2018 – 2028 261
Figure 9 41: Cervical Standalone Interbody Device Market, Japan, 2018 – 2028 262
Figure 9 42: Lumbar Standalone Interbody Device Market, Japan, 2018 – 2028 263
Figure 9 43: Leading Competitors, Interbody Device Market, Japan, 2021 268
Figure 10 1: Vertebral Compression Fracture Market by Segment, Japan, 2018 – 2028 (US$M) 273
Figure 10 2: Vertebral Compression Fracture Market by Segment, Japan, 2018 – 2028 (JP¥M) 274
Figure 10 3: Vertebral Compression Fracture Market, Japan, 2018 – 2028 276
Figure 10 4: Vertebroplasty Market, Japan, 2018 – 2028 277
Figure 10 5: Percutaneous Vertebral Augmentation Market, Japan, 2018 – 2028 278
Figure 10 6: Leading Competitors, Vertebral Compression Fracture Market, Japan, 2021 283
Figure 11 1: Spinal Surgery Instrumentation Market, Japan, 2018 – 2028 287
Figure 11 2: Leading Competitors, Spinal Surgery Instrumentation Market, Japan, 2021 292
Figure 12 1: Spinal Electrical Stimulation Device Market by Segment, Japan, 2018 – 2028 (US$M) 295
Figure 12 2: Spinal Electrical Stimulation Device Market by Segment, Japan, 2018 – 2028 (JP¥M) 296
Figure 12 3: Electrical Stimulation Market, Japan, 2018 – 2028 298
Figure 12 4: Cervical Electrical Stimulation Market, Japan, 2018 – 2028 300
Figure 12 5: Lumbar Spinal Electrical Stimulation Device Market, Japan, 2018 – 2028 302
iData’s 9-Step Research Methodology
Our reports follow an in-depth 9-step methodology which focuses on the following research systems:
- Original primary research that consists of the most up-to-date market data
- Strong foundation of quantitative and qualitative research
- Focused on the needs and strategic challenges of the industry participants
Step 1: Project Initiation & Team Selection During this preliminary investigation, all staff members involved in the industry discusses the topic in detail.
Step 2: Prepare Data Systems and Perform Secondary Research The first task of the research team is to prepare for the data collection process: Filing systems and relational databases are developed as needed.
Step 3: Preparation for Interviews & Questionnaire Design The core of all iData research reports is primary market research. Interviews with industry insiders represent the single most reliable way to obtain accurate, current data about market conditions, trends, threats and opportunities.
Step 4: Performing Primary Research At this stage, interviews are performed using contacts and information acquired in the secondary research phase.
Step 5: Research Analysis: Establishing Baseline Estimates Following the completion of the primary research phase, the collected information must be synthesized into an accurate view of the market status. The most important question is the current state of the market.
Step 6: Market Forecast and Analysis iData Research uses a proprietary method to combine statistical data and opinions of industry experts to forecast future market values.
Step 7: Identify Strategic Opportunities iData analysts identify in broad terms why some companies are gaining or losing share within a given market segment.
Step 8: Final Review and Market Release An integral part of the iData research methodology is a built-in philosophy of quality control and continuing improvement is integral to the iData philosophy.
Step 9: Customer Feedback and Market Monitoring iData philosophy of continuous improvement requires that reports and consulting projects be monitored after release for customer feedback and market accuracy.
Click Here to Read More About Our Methodology
 Throughout this study on Japan’s spinal implants market, iData Research has performed an analysis on cervical fixation procedures, thoracolumbar fixation procedures, interbody fusion procedures, motion preservation procedures, and vertebral compression fracture procedures to increase the accuracy of our market sizing and provide you with the volume of procedures over a 10-year range.
Throughout this study on Japan’s spinal implants market, iData Research has performed an analysis on cervical fixation procedures, thoracolumbar fixation procedures, interbody fusion procedures, motion preservation procedures, and vertebral compression fracture procedures to increase the accuracy of our market sizing and provide you with the volume of procedures over a 10-year range.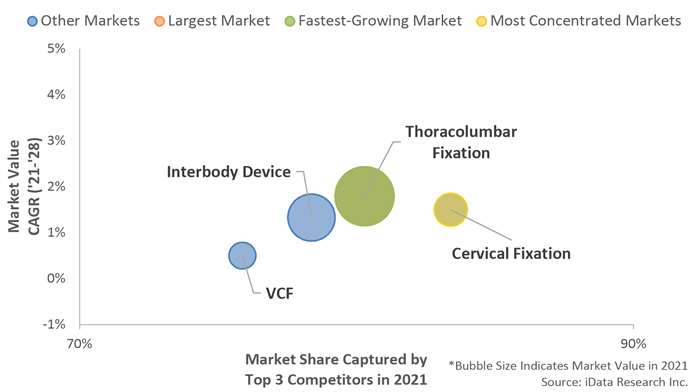 One ongoing trend in the Japan market is favorable demographics. Both fusion and non-fusion procedures are typically performed in order to treat spinal complications that are much more common in elderly patients. As of 2020, approximately 28% of the Japanese population was over 65 years of age. By 2036, those over 65 will comprise over a third of the population. Growth of this population is expected to stimulate growth in both fusion and non-fusion spinal implant markets.
One ongoing trend in the Japan market is favorable demographics. Both fusion and non-fusion procedures are typically performed in order to treat spinal complications that are much more common in elderly patients. As of 2020, approximately 28% of the Japanese population was over 65 years of age. By 2036, those over 65 will comprise over a third of the population. Growth of this population is expected to stimulate growth in both fusion and non-fusion spinal implant markets. Medtronic was the leading competitor in every major segment except for vertebral compression fracture (VCF). The company offers an extensive portfolio in all segments, which has allowed for the company’s continued success within the market. DePuy Synthes was the second-leading competitor in the spinal implant and VCF market. It was the second-leading competitor in all major market segments. The company’s largest market share was in the thoracolumbar fixation market segment.
Medtronic was the leading competitor in every major segment except for vertebral compression fracture (VCF). The company offers an extensive portfolio in all segments, which has allowed for the company’s continued success within the market. DePuy Synthes was the second-leading competitor in the spinal implant and VCF market. It was the second-leading competitor in all major market segments. The company’s largest market share was in the thoracolumbar fixation market segment.






