 Register to receive a free U.S. Market Report Suite for Peripheral Vascular Devices and Accessories – MedSuite report synopsis and brochure
Register to receive a free U.S. Market Report Suite for Peripheral Vascular Devices and Accessories – MedSuite report synopsis and brochure
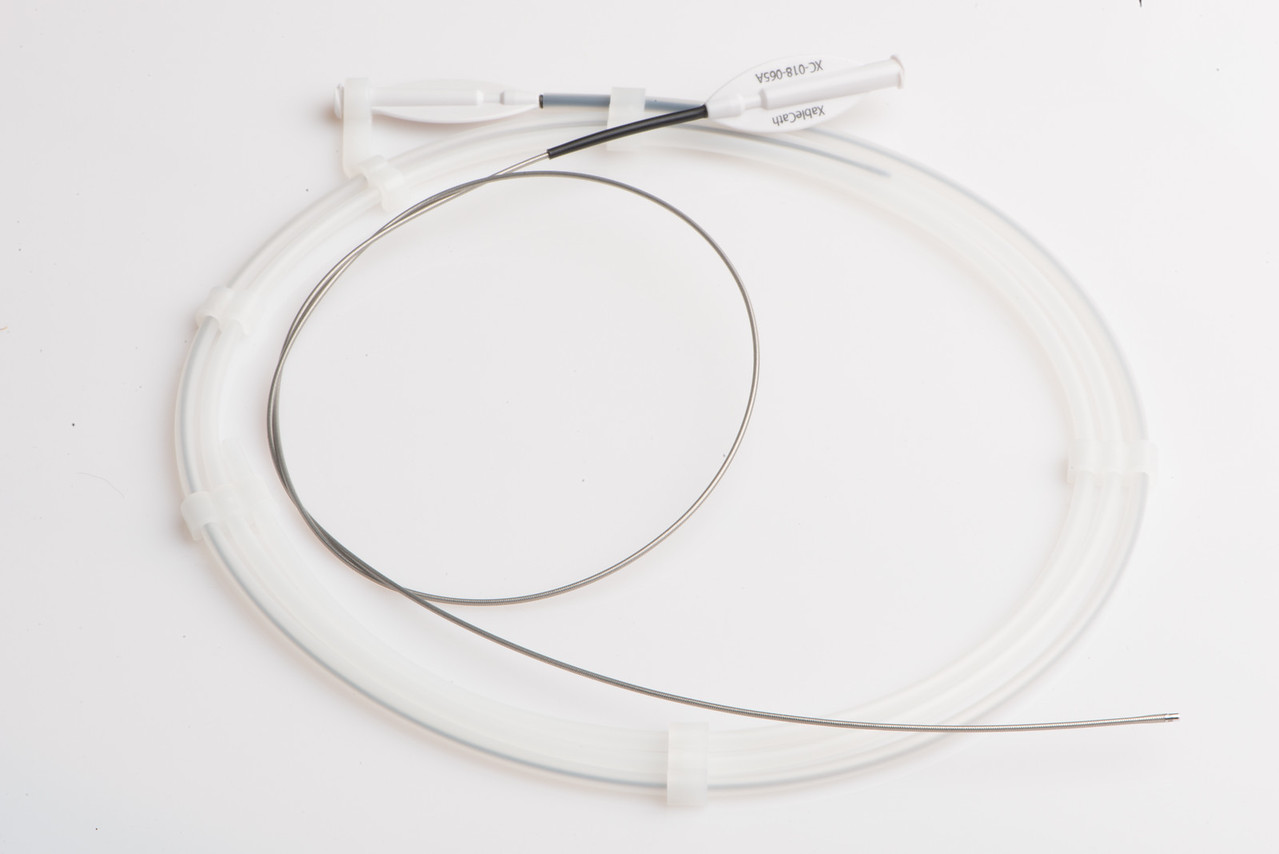
XableCath, Inc., innovators of products designed to aid in the treatment of peripheral artery disease (PAD), announced that it has been granted a second U.S. Patent, No. 9,826,995, covering its XableCath™ catheter. The patent specifically describes a catheter that controls tissue contact and has a rigid ring implement that opens a blood vessel occlusion by rotating the ring against the occlusion, thus clearing the vessel and providing access across the lesion.“Our proprietary design enables the XableCath to easily slide past an occluding atheroma through the true lumen of the vessel,” stated XableCath President and Chief Executive Officer, Lisa Dunlea. “The granting of this second patent provides broad coverage and a strong foundation for XableCath’s intellectual property portfolio and future product development plans.”
Oded Ben-Joseph, PhD, Board Member at XableCath commented, “Following the recent FDA clearance for XableCath’s blunt tip catheter, this new patent provides an additional testimonial for the company’s innovative and unique design to enable a safe and rapid passage through chronic peripheral total occlusions, both above and below the knee. We believe that the ability to treat complex occlusions addresses a clear and unmet clinical need and will ultimately help both patients and physicians.”
Peripheral arterial disease (PAD) is a disease of the arteries that carry blood to the limbs. Diseased arteries narrow or are blocked, thereby restricting adequate blood flow to reach peripheral extremities beyond the obstruction. PAD is the major cause of limb amputations, affecting more than 200 million people worldwide — including 27 million people in Europe and the United States. Most amputations can be prevented with appropriate treatment.
The XableCath support catheter is designed to treat PAD by facilitating over-the-wire passage through the true artery lumen enabling endovascular treatment options such as angioplasty, stenting, or atherectomy. XableCath technology is designed to modify atheroma edges facilitating rapid and safe passage across tough arterial lesions in the peripheral vascular.
The company is on pace to perform its first cases in the United States during the first half of 2018.
About XableCath
XableCath was founded in 2014 to commercialize catheters for the treatment of vascular disease. Its revolutionary technology allows effective treatment, improving the lives of patients and transforming the ease of vascular interventions for physicians.
SALT LAKE CITY –(Business Wire)– http://www.digitaljournal.com/pr/3691328
For Further Information
More on the peripheral vascular stent market in the U.S. can be found in a report series published by iData Research entitled the U.S. Market Report Suite for Peripheral Vascular Devices and Accessories. The suite covers reports on the following markets: Peripheral Vascular Stent Market, PTA Balloon Catheter Market, Atherectomy Device Market, Chronic Total Occlusion (CTO) Device Market, Embolic Protection Device Market, Stent Graft Market, Surgical Graft Market, Diagnostic and Interventional Catheter Market, Diagnostic and Interventional Guidewire Market, Introducer Sheath Market, Inferior Vena Cava Filter Market, Arteriovenous (AV) Access Thrombectomy Device Market, Vascular Closure Device Market, and Transcatheter Embolization Device Market.
Reports also provide a comprehensive analysis including units sold, procedure numbers, market value, forecasts, as well as detailed competitive market shares and analysis of major players’ success strategies in each market and segment. To find out more about peripheral vascular device market data or procedure data, register online or email us at info@idataresearch.net for a U.S. Market Report Suite for Peripheral Vascular Devices and Accessories brochure and synopsis.
 Register to receive a free
Register to receive a free