 Register to receive a free US Orthopedic Large Joint Replacement Device Market Report Suite synopsis and brochure
Register to receive a free US Orthopedic Large Joint Replacement Device Market Report Suite synopsis and brochure
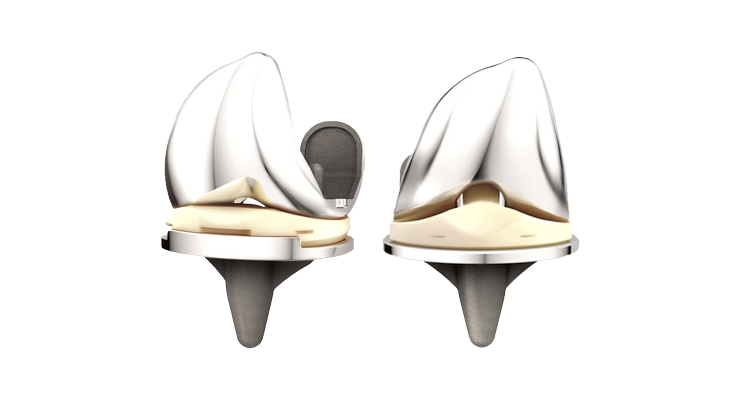
DePuy Synthes, part of the Johnson & Johnson Medical Devices Companies, announced the US launch of the ATTUNE® Revision Knee System, to complement the ATTUNE Primary Knee System and provide surgeons with a comprehensive solution for knee replacement. The ATTUNE Revision Knee System was introduced at the 2018 American Academy of Orthopaedic Surgeons (AAOS) Annual Meeting in New Orleans, LA.
While knee replacement is widely recognized as one of the most common and successful surgical procedures, approximately five percent of patients will require a revision surgery 10 years after the initial procedure for a variety of reasons. Revision surgeries can create complexities particularly in cases where large bone defects present reconstruction and fixation challenges. The ATTUNE Revision Knee System aims to address some of those challenges through improved kinematics, fixation and patient fit. With both fixed-bearing and rotating platform options, it is one of the most comprehensive revision knee systems available on the market today.
“The ATTUNE Revision Knee System provides the implant options I need to treat a variety of patient anatomies including more metaphyseal sleeve sizes, offset adaptors, stems and fixation choices,” said Dr. Douglas Dennis, Orthopaedic Surgeon, Denver, CO. “The ATTUNE Revision Knee System incorporates the advantages of the ATTUNE Primary Knee System such as AOX™ Polyethylene, enhanced locking mechanisms and improved kinematics via the ATTUNE GRADIUS™ Curve condylar geometry.”
In a large, multi-center study, the ATTUNE Primary Knee System demonstrated improved outcomes across a broad range of Patient Reported Outcome Measures (PROMs) compared to certain existing knee brands at one-year minimum follow-up. The same technologies that helped deliver these outcomes are also incorporated in the ATTUNE Revision Knee System, helping to address overall patient satisfaction.
“The ATTUNE Revision Knee System introduces a new chapter in the ATTUNE Knee story,” said Rajit Kamal, Vice President and Global Platform Leader, Knees, DePuy Synthes. “With six years of successful clinical history and more than 575,000 ATTUNE Primary Knees implanted worldwide, the new ATTUNE Revision Knee System allows us to bring proprietary technologies of the ATTUNE Knee System to more patients around the world.”
The ATTUNE Revision Knee System is designed to work in harmony with the patient’s anatomy to deliver stability in motion during activities such as stair ascent or descent, walking uphill or downhill or getting up from a chair through:
- – ATTUNE GRADIUS Curve is designed to address the unnatural sliding of the femur on the tibia, to provide smooth motion and stability during everyday activities
- – GLIDERIGHT™ Articulation is designed to enable the ATTUNE Knee design to more accurately replicate the normal patello-femoral kinematics of the native knee
The ATTUNE Revision Knee System is now available in the US.
About the Johnson & Johnson Medical Devices Companies
The Johnson & Johnson Medical Devices Companies** have been working to make surgery better for more than a century. With substantial breadth and depth in surgical and orthopaedic technologies and interventional solutions, we aspire to improve and enhance medical care for people worldwide. Together, we are working to shape the future of health through differentiated products and services.
About DePuy Synthes
DePuy Synthes, part of the Johnson & Johnson Medical Devices Companies, provides one of the most comprehensive orthopaedics portfolios in the world. DePuy Synthes solutions, in specialties including joint reconstruction, trauma, craniomaxillofacial, spinal surgery and sports medicine, are designed to advance patient care while delivering clinical and economic value to health care systems worldwide.
For Further Information
More on the orthopedic large joints replacement market in the U.S. can be found in a series of reports published by iData entitled the U.S. Market Report Suite for Orthopedic Large Joints Replacement Devices. This report covers the following market segments: hip implants, knee implants, and bone cement.
The iData series on the market for orthopedic large joint replacement devices covers the U.S., India, Japan, China, and 15 countries in Europe including Germany, France, the United Kingdom (U.K.), Italy, Spain, Benelux (Belgium, Netherlands and Luxemburg), Scandinavia (Finland, Denmark, Sweden and Norway), Portugal, Austria and Switzerland. Reports provide a comprehensive analysis including units sold, procedure numbers, market value, forecasts, as well as detailed competitive market shares and analysis of major players’ success strategies in each market and segment. To find out more about orthopedics large joint replacement market data or procedure data, register online or email us at info@idataresearch.net for an U.S. Market Report Suite for Orthopedic Large Joints Replacement Devices brochure and synopsis.
 Register to receive a free
Register to receive a free