Register to receive a free U.S. Market Report Suite for Gastrointestinal Endoscopic Devices – MedSuite report synopsis and brochure
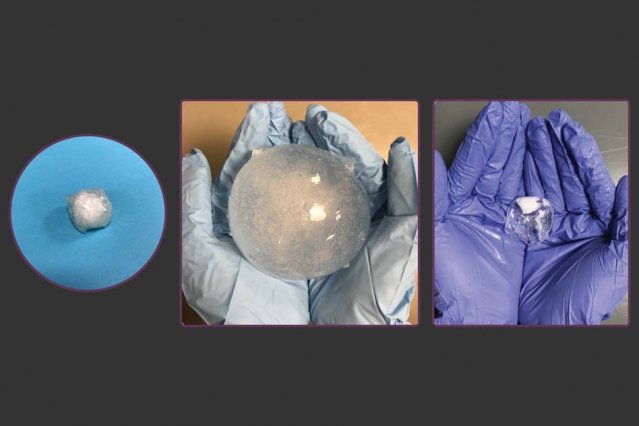
Engineers at MIT have developed an inflatable ingestible capsule for continuous long-term stomach monitoring. The pill’s materials are designed such that it can be easily swallowed by the patient and inflate quickly in the stomach to a size that is too large to pass out of the stomach through the pylorus. Xuanhe Zhao, associate professor of mechanical engineering at MIT and senior collaborator Giovanni Traverso, a visiting scientist who will join the MIT faculty in 2019, along with lead authors Xinyue Liu, Christoph Steiger, and Shaoting Lin, have published their results in Nature Communications.
The device is composed of sodium polyacrylate, an absorbent, expandable material found in commercial products such as diapers as they can quickly soak up liquid and inflate. However, due to the nature of this material, a device made of it alone would not be structurally sound enough to endure the conditions of the stomach. As such, the engineers developed an outer protective hydrogel layer made of nanoscopic crystalline chains, providing excellent mechanical and structural strength. The hydrogel can not only withstand thousands of tests for stomach conditions, but any holes manually applied to the material do not grow when subject to mechanical testing.
“The stomach applies thousands to millions of cycles of load to grind food down,” Shaoting Lin of the lead authors explains. “And we found that even when we make a small cut in the membrane, and then stretch and squeeze it thousands of times, the cut does not grow larger. Our design is very robust.”
The capsule can be deflated by the ingestion of a solution of calcium ions, which shrinks the swollen particles. Once reduced in size, the pill is small enough to pass out the stomach.
Currently tested in pigs, the pill has the capacity to continuously monitor temperature measurements over time. The sensor was able to correctly track the animals’ daily activity patterns for up to a month. Researchers hope to apply different monitoring techniques to this capsule technology in the future, for instance, pH level monitoring or diagnoses for certain bacteria or viruses. Small cameras could also be embedded into the device to image tumor development progress or ulcer tracking over the course of several weeks. This gives a new, longer-term approach to traditional capsule endoscopy, which is used commonly today.
Market research published by iData research finds that the U.S. capsule endoscopy market has been experiencing significant growth, with a CAGR of just under 6%. Its usefulness in the esophageal and colonic market are driving its stable growth rates, which are expected to increase steadily over the forecast period. Minimal patient risk and physician training requirements have already created a healthy environment for this market segment, and additional innovations such as MIT’s long-term monitoring developments are projected to propel this market further.
For Further Information
More on the GI endoscopy market in the U.S. can be found in a series of reports published by iData Research entitled the U.S. Market Report Suite for Gastrointestinal Endoscopic Devices.