| Figure 1‑1: Gastrointestinal Endoscopic Device Market Share Ranking by Segment, Europe, 2021 (1 of 2) |
| Figure 1‑2: Gastrointestinal Endoscopic Device Market Share Ranking by Segment, Europe, 2021 (2 of 2) |
| Figure 1‑3: Companies Researched in this Report |
| Figure 1‑4: Factors Impacting the Gastrointestinal Endoscopic Device Market by Segment, Europe (1 of 2) |
| Figure 1‑5: Factors Impacting the Gastrointestinal Endoscopic Device Market by Segment, Europe (2 of 2) |
| Figure 1‑6: Recent Events in the Gastrointestinal Endoscopic Device Market, Europe, 2018 – 2021 |
| Figure 1‑7: Gastrointestinal Endoscopic Device Procedures Covered, Europe |
| Figure 1‑8: Gastrointestinal Endoscopic Device Markets Covered, Europe (1 of 3) |
| Figure 1‑9: Gastrointestinal Endoscopic Device Markets Covered, Europe (2 of 3) |
| Figure 1‑10: Gastrointestinal Endoscopic Device Markets Covered, Europe (3 of 3) |
| Figure 1‑11: Key Report Updates |
| Figure 1‑12: Version History |
| Figure 2‑1: Gastrointestinal Endoscopic Device Market by Segment, Worst Case Scenario, Europe, 2018 – 2028 (€M) (1 of 2) |
| Figure 2‑2: Gastrointestinal Endoscopic Device Market by Segment, Worst Case Scenario, Europe, 2018 – 2028 (€M) (2 of 2) |
| Figure 2‑3: Gastrointestinal Endoscopic Device Market by Segment, Base Case Scenario, Europe, 2018 – 2028 (€M) (1 of 2) |
| Figure 2‑4: Gastrointestinal Endoscopic Device Market by Segment, Worst Case Scenario, Europe, 2018 – 2028 (€M) (2 of 2) |
| Figure 2‑5: Gastrointestinal Endoscopic Device Market by Segment, Best Case Scenario, Europe, 2018 – 2028 (€M) (1 of 2) |
| Figure 2‑6: Gastrointestinal Endoscopic Device Market by Segment, Best Case Scenario, Europe, 2018 – 2028 (€M) (2 of 2) |
| Figure 4‑1: FIT-DNA Test Devices by Company |
| Figure 4‑2: Gastrointestinal Endoscopy by Company (1 of 4) |
| Figure 4‑3: Gastrointestinal Endoscopy by Company (2 of 4) |
| Figure 4‑4: Gastrointestinal Endoscopy by Company (3 of 4) |
| Figure 4‑5: Gastrointestinal Endoscopy by Company (4 of 4) |
| Figure 4‑6: Capsule Endoscopy by Company |
| Figure 4‑7: Virtual Colonoscopy by Company |
| Figure 4‑8: Endoscope Distal Attachment Cap by Company |
| Figure 4‑9: Endoscopic Submucosal Dissection Knives by Company |
| Figure 4‑10: Stenting and Dilation Devices by Company (1 of 2) |
| Figure 4‑11: Stenting and Dilation Devices by Company (2 of 2) |
| Figure 4‑12: Submucosal Lifting Agents by Company |
| Figure 4‑13: ERCP Devices by Company (1 of 4) |
| Figure 4‑14: ERCP Devices by Company (2 of 4) |
| Figure 4‑15: ERCP Devices by Company (3 of 4) |
| Figure 4‑16: ERCP Devices by Company (4 of 4) |
| Figure 4‑17: Biopsy Forceps, Polypectomy Snares, Fine Aspiration Needles by Company |
| Figure 4‑18: Foreign Body Retrieval and Specimen Removal Devices by Company (1 of 2) |
| Figure 4‑19: Foreign Body Retrieval and Specimen Removal Devices by Company (2 of 2) |
| Figure 4‑20: Hemostasis Devices by Company |
| Figure 4‑21: Enteral Feeding Devices by Company (1 of 3) |
| Figure 4‑22: Enteral Feeding Devices by Company (2 of 3) |
| Figure 4‑23: Enteral Feeding Devices by Company (3 of 3) |
| Figure 4‑24: Anti-Reflux Devices by Company |
| Figure 4‑25: Barrett’s Esophagus Ablation Devices by Company |
| Figure 4‑26: Class 2 Device Recall CORFLO Percutaneous Endoscopic Gastrostomy (PEG) Kit |
| Figure 4‑27: Class 2 Device Recall CORFLO Percutaneous Endoscopic Gastrostomy (PEG)Kit |
| Figure 4‑28: Class 2 Device Recall AVANOS MICKEY SF Gastrostomy Feeding Tube |
| Figure 4‑29: Class 2 Device Recall CORFLO NG/NI Pediatric Neonatal Feeding Tube w/ AntiIV Connector |
| Figure 4‑30: Class 2 Device Recall Captivator Polypectomy Snare 27mm, Hexagonal Loop |
| Figure 4‑31: Class 2 Device Recall WallFlexTM Biliary RX Fully Covered Stent System RMV |
| Figure 4‑32: Class 2 Device Recall ConMed Anchor Tissue Retrieval System 10 MM |
| Figure 4‑33: Class 2 Device Recall Captura Serrated Forceps with Spike |
| Figure 4‑34: Class 2 Device Recall Captura Serrated Large Forcep |
| Figure 4‑35: Class 2 Device Recall EVIS EXERA Duodenovideoscopes OLYMPUS JF140F |
| Figure 4‑36: Class 2 Device Recall Ultrasound Gastroscopes, Gastroscopes, Colonoscopes and Duodenoscopes |
| Figure 4‑37: Class 2 Device Recall Olympus CHFCB30S Choledoscope |
| Figure 4‑38: Class 2 Device Recall Ultrasonic Gastrovideoscope/ Endoscopic Ultrasound Endoscopes |
| Figure 4‑39: Class 2 Device Recall Pentax Video Duodenoscope |
| Figure 4‑40: Class 2 Device Recall Video Colonoscope EC34i10L Video Colonoscope EC38i10L |
| Figure 4‑41: Class 2 Device Recall LINX Reflux Management System |
| Figure 4‑42: Development of Applications of PillCam™ Endoscopy and Patency Systems and Clinical Evaluation of Their Performance in Healthy Volunteers ("HEIGHT" Study) |
| Figure 4‑43: Study of Early Endocapsule (EC) in Clinical Decision Unit Versus Standard of Care Work-up for GI Bleeding |
| Figure 4‑44: Evaluation of Obscure Gastrointestinal Bleeding Patients With Conventional Capsule Endoscopy and Panoramic Side View Capsule Endoscopy |
| Figure 4‑45: AXIOS™ for Gallbladder Drainage as an Alternative to Percutaneous Drainage IDE |
| Figure 4‑46: Plastic vs. Fully Covered Self Expanding Stents (FCSEMS) for Treatment of Anastomotic Bile Leaks |
| Figure 4‑47: Self-expandable Metal Stents Versus Multiple Plastic Stents for Palliation of Biliary Obstruction |
| Figure 4‑48: Intraintestinal Extended Biliary Stents Preventing Duodenobiliary Reflux in Patients With Biliary Stricture |
| Figure 4‑49: Three Arm Rand Trial of HD Light vs Reveal® Cap vs Endocuff Cap for Detection of Colorectal Adenomas (TRACE) (TRACE) |
| Figure 4‑50: Initial Case Series With Exalt Single-Use Duodenoscope |
| Figure 4‑51: Global Prospective Case Series Using a Single-Use Duodenoscope |
| Figure 4‑52: Reusable Versus Disposable Duodenoscopes for ERCP |
| Figure 4‑53: Single Use ERCP -SURE Study (SURE) |
| Figure 4‑54: Monopolar Current Cutting Knife vs Bipolar RFA Knife |
| Figure 4‑55: Effectiveness of Multi-target FIT-DNA Analysis as a Colorectal Cancer Screening Test |
| Figure 4‑56: Comparison of Using Regular or Rotary Polypectomy Snare for the Resection of Colorectal Polyps |
| Figure 4‑57: Fine Needle Biopsy of Solid Pancreatic Mass Lesions |
| Figure 4‑58: The Use of Over-the-scope-clip for Prevention of Rebleeding in High Risk Peptic Ulcers |
| Figure 4‑59: Carvedilol Versus Endoscopic Band Ligation for Primary Prophylaxis of Oesophageal Variceal Bleeding |
| Figure 4‑60: The Effect of Rubber Band Ligation in Bleeding Internal Hemorrhoids. |
| Figure 4‑61: Optimal Feeding Tube Placement |
| Figure 4‑62: Feasibility Study to Evaluate Safety of ENvizion Medical™ ENVUE™ System |
| Figure 4‑63: Comparative Study Between Two Routes of Enteral Feeding |
| Figure 4‑64: LINX Reflux Management System or Fundoplication Clinical Study in Patients With Hiatal Hernia >3 cm |
| Figure 5‑1: Currency Exchange Rate, 2021 |
| Figure 5‑2: Gastrointestinal Endoscopic Device Market by Segment, Europe, 2018 – 2028 (€M) (1 of 2) |
| Figure 5‑3: Gastrointestinal Endoscopic Device Market by Segment, Europe, 2018 – 2028 (€M) (2 of 2) |
| Figure 5‑4: Gastrointestinal Endoscopic Device Market by Segment, Europe, 2018 – 2028 (US$M) (1 of 2) |
| Figure 5‑5: Gastrointestinal Endoscopic Device Market by Segment, Europe, 2018 – 2028 (US$M) (2 of 2) |
| Figure 5‑6: Leading Competitors, Gastrointestinal Endoscopic Device Market, Europe, 2021 (1 of 2) |
| Figure 5‑7: Leading Competitors, Gastrointestinal Endoscopic Device Market, Europe, 2021 (2 of 2) |
| Figure 5‑8: SWOT Analysis, Avanos Medical |
| Figure 5‑9: SWOT Analysis, Boston Scientific |
| Figure 5‑10: SWOT Analysis, Cardinal Health |
| Figure 5‑11: SWOT Analysis, CONMED |
| Figure 5‑12: SWOT Analysis, Cook Medical |
| Figure 5‑13: SWOT EndoGastric Solutions |
| Figure 5‑14: SWOT Analysis, ERBE |
| Figure 5‑15: SWOT Analysis, Exact Sciences |
| Figure 5‑16: SWOT Analysis, Fujifilm |
| Figure 5‑17: SWOT Analysis, GE Healthcare |
| Figure 5‑18: SWOT Analysis, Johnson & Johnson |
| Figure 5‑19: SWOT Analysis, Medline |
| Figure 5‑20: SWOT Analysis, Medtronic (1 of 2) |
| Figure 5‑21: SWOT Analysis, Medtronic (2 of 2) |
| Figure 5‑22: SWOT Analysis, Moog |
| Figure 5‑23: SWOT Analysis, Olympus |
| Figure 5‑24: SWOT Analysis, Pentax |
| Figure 5‑25: SWOT Analysis, STERIS |
| Figure 7‑1: Gastrointestinal Procedures by Segment, Europe, 2018 – 2028 (1 of 3) |
| Figure 7‑2: Gastrointestinal Procedures by Segment, Europe, 2018 – 2028 (2 of 2) |
| Figure 7‑3: Fit-DNA Test Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑4: Gastrointestinal Endoscopy Procedures by Segment, Europe, 2018 – 2028 |
| Figure 7‑5: Gastrointestinal Endoscopy Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑6: EGD Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑7: Colonoscopy Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑8: Sigmoidoscopy Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑9: Enteroscopy Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑10: Ultrasound Endoscopy Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑11: ERCP Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑12: Capsule Endoscopy Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑13: Stenting Procedures by Segment, Europe, 2018 – 2028 |
| Figure 7‑14: Stenting Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑15: Esophageal Stenting Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑16: Duodenal Stenting Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑17: Colonic Stenting Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑18: Biliary and Pancreatic Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑19: Dilation Procedures by Segment, Europe, 2018 – 2028 |
| Figure 7‑20: Dilation Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑21: Esophageal Dilation Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑22: Duodenal Dilation Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑23: Colonic Dilation Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑24: Biliary and Pancreatic Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑25: Tissue Biopsy Procedures by Segment, Europe, 2015 – 2025 |
| Figure 7‑26: Tissue Biopsy Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑27: Biopsy Forceps Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑28: EUS Needle Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑29: Polypectomy Snare Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑30: Hemostasis Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑31: Enteral Feeding Tube Placement Procedures by Country, Europe, 2018 – 2028 |
| Figure 7‑32: Anti-Reflux Device Implantation Procedures by Country, Europe, 2018 – 2028 |
| Figure 8‑1: FIT-DNA Test Market, Europe, 2018 – 2028 |
| Figure 8‑2: Leading Competitors, FIT-DNA Test Market, Europe, 2021 |
| Figure 9‑1: Gastrointestinal Endoscope Market by Segment, Europe, 2018 – 2028 (€M) |
| Figure 9‑2: Gastrointestinal Endoscope Market by Segment, Europe, 2018 – 2028 (US$M) |
| Figure 9‑3: New Reusable Gastrointestinal Endoscope Market by Segment, Europe, 2018 – 2028 (€M) |
| Figure 9‑4: New Reusable Gastrointestinal Endoscope Market by Segment, Europe, 2018 – 2028 (US$M) |
| Figure 9‑5: New Reusable Gastrointestinal Endoscope Market, Europe, 2018 – 2028 |
| Figure 9‑6: Units Sold by Country, New Reusable Gastrointestinal Endoscope Market, Europe, 2018 – 2028 |
| Figure 9‑7: Average Selling Price by Country, New Reusable Gastrointestinal Endoscope Market, Europe, 2018 – 2028 (€) |
| Figure 9‑8: Average Selling Price by Country, New Reusable Gastrointestinal Endoscope Market, Europe, 2018 – 2028 (US$) |
| Figure 9‑9: Market Value by Country, New Reusable Gastrointestinal Endoscope Market, Europe, 2018 – 2028 (€M) |
| Figure 9‑10: Market Value by Country, New Reusable Gastrointestinal Endoscope Market, Europe, 2018 – 2028 (US$M) |
| Figure 9‑11: New Reusable Colonoscope Market, Europe, 2018 – 2028 |
| Figure 9‑12: Units Sold by Country, New Reusable Colonoscope Market, Europe, 2018 – 2028 |
| Figure 9‑13: Average Selling Price by Country, New Reusable Colonoscope Market, Europe, 2018 – 2028 (€) |
| Figure 9‑14: Average Selling Price by Country, New Reusable Colonoscope Market, Europe, 2018 – 2028 (US$) |
| Figure 9‑15: Market Value by Country, New Reusable Colonoscope Market, Europe, 2018 – 2028 (€M) |
| Figure 9‑16: Market Value by Country, New Reusable Colonoscope Market, Europe, 2018 – 2028 (US$M) |
| Figure 9‑17: New Reusable Duodenoscope Market, Europe, 2018 – 2028 |
| Figure 9‑18: Units Sold by Country, New Reusable Duodenoscope Market, Europe, 2018 – 2028 |
| Figure 9‑19: Average Selling Price by Country, New Reusable Duodenoscope Market, Europe, 2018 – 2028 (€) |
| Figure 9‑20: Average Selling Price by Country, New Reusable Duodenoscope Market, Europe, 2018 – 2028 (US$) |
| Figure 9‑21: Market Value by Country, New Reusable Duodenoscope Market, Europe, 2018 – 2028 (€M) |
| Figure 9‑22: Market Value by Country, New Reusable Duodenoscope Market, Europe, 2018 – 2028 (US$M) |
| Figure 9‑23: New Reusable Enteroscope Market, Europe, 2018 – 2028 |
| Figure 9‑24: Units Sold by Country, New Reusable Enteroscope Market, Europe, 2018 – 2028 |
| Figure 9‑25: Average Selling Price by Country, New Reusable Enteroscope Market, Europe, 2018 – 2028 (€) |
| Figure 9‑26: Average Selling Price by Country, New Reusable Enteroscope Market, Europe, 2018 – 2028 (US$) |
| Figure 9‑27: Market Value by Country, New Reusable Enteroscope Market, Europe, 2018 – 2028 (€M) |
| Figure 9‑28: Market Value by Country, New Reusable Enteroscope Market, Europe, 2018 – 2028 (US$M) |
| Figure 9‑29: New Reusable Ultrasound Endoscope Market, Europe, 2018 – 2028 |
| Figure 9‑30: Units Sold by Country, New Reusable Ultrasound Endoscope Market, Europe, 2018 – 2028 |
| Figure 9‑31: Average Selling Price by Country, New Reusable Ultrasound Endoscope Market, Europe, 2018 – 2028 (€) |
| Figure 9‑32: Average Selling Price by Country, New Reusable Ultrasound Endoscope Market, Europe, 2018 – 2028 (US$) |
| Figure 9‑33: Market Value by Country, New Reusable Ultrasound Endoscope Market, Europe, 2018 – 2028 (€M) |
| Figure 9‑34: Market Value by Country, New Reusable Ultrasound Endoscope Market, Europe, 2018 – 2028 (US$M) |
| Figure 9‑35: New Reusable Gastroscope Market, Europe, 2018 – 2028 |
| Figure 9‑36: Units Sold by Country, New Reusable Gastroscopes Market, Europe, 2018 – 2028 |
| Figure 9‑37: Average Selling Price by Country, New Reusable Gastroscopes Market, Europe, 2018 – 2028 (€) |
| Figure 9‑38: Average Selling Price by Country, New Reusable Gastroscopes Market, Europe, 2018 – 2028 (US$) |
| Figure 9‑39: Market Value by Country, New Reusable Gastroscopes Market, Europe, 2018 – 2028 (€M) |
| Figure 9‑40: Market Value by Country, New Reusable Gastroscopes Market, Europe, 2018 – 2028 (US$M) |
| Figure 9‑41: Single-Use Duodenoscope Market, Europe, 2018 – 2028 |
| Figure 9‑42: Units Sold by Country, Single-Use Duodenoscope Market, Europe, 2018 – 2028 |
| Figure 9‑43: Average Selling Price by Country, Single-Use Duodenoscope Market, Europe, 2018 – 2028 (€) |
| Figure 9‑44: Average Selling Price by Country, Single-Use Duodenoscope Market, Europe, 2018 – 2028 (US$) |
| Figure 9‑45: Market Value by Country, Single-Use Duodenoscope Market, Europe, 2018 – 2028 (€M) |
| Figure 9‑46: Market Value by Country, Single-Use Duodenoscope Market, Europe, 2018 – 2028 (US$M) |
| Figure 9‑47: Leading Competitors, Gastrointestinal Endoscope Market, Europe, 2021 |
| Figure 9‑48: Leading Competitors by Country, New Reusable Gastrointestinal Endoscope Market, Europe, 2021 |
| Figure 10‑1: Camera Capsule Market, Europe, 2018 – 2028 |
| Figure 10‑2: Units Sold by Country, Camera Capsule Market, Europe, 2018 – 2028 |
| Figure 10‑3: Average Selling Price by Country, Camera Capsule Market, Europe, 2018 – 2028 (€) |
| Figure 10‑4: Average Selling Price by Country, Camera Capsule Market, Europe, 2018 – 2028 (US$) |
| Figure 10‑5: Market Value by Country, Camera Capsule Market, Europe, 2018 – 2028 (€M) |
| Figure 10‑6: Market Value by Country, Camera Capsule Market, Europe, 2018 – 2028 (US$M) |
| Figure 10‑7: Leading Competitors, Capsule Endoscopy Market, Europe, 2021 |
| Figure 11‑1: Stenting and Dilation Device Market by Segment, Europe, 2018 – 2028 (€M) |
| Figure 11‑2: Stenting and Dilation Device Market by Segment, Europe, 2018 – 2028 (US$M) |
| Figure 11‑3: Esophageal Stent Market by Segment, Europe, 2018 – 2028 (€M) |
| Figure 11‑4: Esophageal Stent Market by Segment, Europe, 2018 – 2028 (US$M) |
| Figure 11‑5: Total Esophageal Stent Market, Europe, 2018 – 2028 |
| Figure 11‑6: Units Sold by Country, Total Esophageal Stent Market, Europe, 2018 – 2028 |
| Figure 11‑7: Average Selling Price by Country, Total Esophageal Stent Market, Europe, 2018 – 2028 (€) |
| Figure 11‑8: Average Selling Price by Country, Total Esophageal Stent Market, Europe, 2018 – 2028 (US$) |
| Figure 11‑9: Market Value by Country, Total Esophageal Stent Market, Europe, 2018 – 2028 (€M) |
| Figure 11‑10: Market Value by Country, Total Esophageal Stent Market, Europe, 2018 – 2028 (US$M) |
| Figure 11‑11: Covered-Metal Esophageal Stent Market, Europe, 2018 – 2028 |
| Figure 11‑12: Units Sold by Country, Covered-Metal Esophageal Stent Market, Europe, 2018 – 2028 |
| Figure 11‑13: Average Selling Price by Country, Covered-Metal Esophageal Stent Market, Europe, 2018 – 2028 (€) |
| Figure 11‑14: Average Selling Price by Country, Covered-Metal Esophageal Stent Market, Europe, 2018 – 2028 (US$) |
| Figure 11‑15: Market Value by Country, Covered-Metal Esophageal Stent Market, Europe, 2018 – 2028 (€M) |
| Figure 11‑16: Market Value by Country, Covered-Metal Esophageal Stent Market, Europe, 2018 – 2028 (US$M) |
| Figure 11‑17: Bare-Metal Esophageal Stent Market, Europe, 2018 – 2028 |
| Figure 11‑18: Units Sold by Country, Bare-Metal Esophageal Stent Market, Europe, 2018 – 2028 |
| Figure 11‑19: Average Selling Price by Country, Bare-Metal Esophageal Stent Market, Europe, 2018 – 2028 (€) |
| Figure 11‑20: Average Selling Price by Country, Bare-Metal Esophageal Stent Market, Europe, 2018 – 2028 (US$) |
| Figure 11‑21: Market Value by Country, Bare-Metal Esophageal Stent Market, Europe, 2018 – 2028 (€M) |
| Figure 11‑22: Market Value by Country, Bare-Metal Esophageal Stent Market, Europe, 2018 – 2028 (US$M) |
| Figure 11‑23: Plastic Esophageal Stent Market, Europe, 2018 – 2028 |
| Figure 11‑24: Units Sold by Country, Plastic Esophageal Stent Market, Europe, 2018 – 2028 |
| Figure 11‑25: Average Selling Price by Country, Plastic Esophageal Stent Market, Europe, 2018 – 2028 (€) |
| Figure 11‑26: Average Selling Price by Country, Plastic Esophageal Stent Market, Europe, 2018 – 2028 (US$) |
| Figure 11‑27: Market Value by Country, Plastic Esophageal Stent Market, Europe, 2018 – 2028 (€M) |
| Figure 11‑28: Market Value by Country, Plastic Esophageal Stent Market, Europe, 2018 – 2028 (US$M) |
| Figure 11‑29: Duodenal Stent Market, Europe, 2018 – 2028 |
| Figure 11‑30: Units Sold by Country, Duodenal Stent Market, Europe, 2018 – 2028 |
| Figure 11‑31: Average Selling Price by Country, Duodenal Stent Market, Europe, 2018 – 2028 (€) |
| Figure 11‑32: Average Selling Price by Country, Duodenal Stent Market, Europe, 2018 – 2028 (US$) |
| Figure 11‑33: Market Value by Country, Duodenal Stent Market, Europe, 2018 – 2028 (€M) |
| Figure 11‑34: Market Value by Country, Duodenal Stent Market, Europe, 2018 – 2028 (US$M) |
| Figure 11‑35: Colonic Stent Market, Europe, 2018 – 2028 |
| Figure 11‑36: Units Sold by Country, Colonic Stent Market, Europe, 2018 – 2028 |
| Figure 11‑37: Average Selling Price by Country, Colonic Stent Market, Europe, 2018 – 2028 (€) |
| Figure 11‑38: Average Selling Price by Country, Colonic Stent Market, Europe, 2018 – 2028 (US$) |
| Figure 11‑39: Market Value by Country, Colonic Stent Market, Europe, 2018 – 2028 (€M) |
| Figure 11‑40: Market Value by Country, Colonic Stent Market, Europe, 2018 – 2028 (US$M) |
| Figure 11‑41: Esophageal Dilation Balloon Market by Segment, Europe, 2018 – 2028 (€M) |
| Figure 11‑42: Esophageal Dilation Balloon Market by Segment, Europe, 2018 – 2028 (US$M) |
| Figure 11‑43: Total Esophageal Dilation Balloon Market, Europe, 2018 – 2028 |
| Figure 11‑44: Units Sold by Country, Total Esophageal Dilation Balloon Market, Europe, 2018 – 2028 |
| Figure 11‑45: Average Selling Price by Country, Total Esophageal Dilation Balloon Market, Europe, 2018 – 2028 (€) |
| Figure 11‑46: Average Selling Price by Country, Total Esophageal Dilation Balloon Market, Europe, 2018 – 2028 (US$) |
| Figure 11‑47: Market Value by Country, Total Esophageal Dilation Balloon Market, Europe, 2018 – 2028 (€M) |
| Figure 11‑48: Market Value by Country, Total Esophageal Dilation Balloon Market, Europe, 2018 – 2028 (US$M) |
| Figure 11‑49: Mulit-Stage Esophageal Dilation Balloon Market, Europe, 2018 – 2028 |
| Figure 11‑50: Units Sold by Country, Mulit-Stage Esophageal Dilation Balloon Market, Europe, 2018 – 2028 |
| Figure 11‑51: Average Selling Price by Country, Mulit-Stage Esophageal Dilation Balloon Market, Europe, 2018 – 2028 (€) |
| Figure 11‑52: Average Selling Price by Country, Mulit-Stage Esophageal Dilation Balloon Market, Europe, 2018 – 2028 (US$) |
| Figure 11‑53: Market Value by Country, Mulit-Stage Esophageal Dilation Balloon Market, Europe, 2018 – 2028 (€M) |
| Figure 11‑54: Market Value by Country, Mulit-Stage Esophageal Dilation Balloon Market, Europe, 2018 – 2028 (US$M) |
| Figure 11‑55: Single-Stage Esophageal Dilation Balloon Market, Europe, 2018 – 2028 |
| Figure 11‑56: Units Sold by Country, Single-Stage Esophageal Dilation Balloon Market, Europe, 2018 – 2028 |
| Figure 11‑57: Average Selling Price by Country, Single-Stage Esophageal Dilation Balloon Market, Europe, 2018 – 2028 (€) |
| Figure 11‑58: Average Selling Price by Country, Single-Stage Esophageal Dilation Balloon Market, Europe, 2018 – 2028 (US$) |
| Figure 11‑59: Market Value by Country, Single-Stage Esophageal Dilation Balloon Market, Europe, 2018 – 2028 (€M) |
| Figure 11‑60: Market Value by Country, Single-Stage Esophageal Dilation Balloon Market, Europe, 2018 – 2028 (US$M) |
| Figure 11‑61: Duodenal Dilation Balloon Market, Europe, 2018 – 2028 |
| Figure 11‑62: Units Sold by Country, Duodenal Dilation Balloon Market, Europe, 2018 – 2028 |
| Figure 11‑63: Average Selling Price by Country, Duodenal Dilation Balloon Market, Europe, 2018 – 2028 (€) |
| Figure 11‑64: Average Selling Price by Country, Duodenal Dilation Balloon Market, Europe, 2018 – 2028 (US$) |
| Figure 11‑65: Market Value by Country, Duodenal Dilation Balloon Market, Europe, 2018 – 2028 (€M) |
| Figure 11‑66: Market Value by Country, Duodenal Dilation Balloon Market, Europe, 2018 – 2028 (US$M) |
| Figure 11‑67: Colonic Dilation Balloon Market, Europe, 2018 – 2028 |
| Figure 11‑68: Units Sold by Country, Colonic Dilation Balloon Market, Europe, 2018 – 2028 |
| Figure 11‑69: Average Selling Price by Country, Colonic Dilation Balloon Market, Europe, 2018 – 2028 (€) |
| Figure 11‑70: Average Selling Price by Country, Colonic Dilation Balloon Market, Europe, 2018 – 2028 (US$) |
| Figure 11‑71: Market Value by Country, Colonic Dilation Balloon Market, Europe, 2018 – 2028 (€M) |
| Figure 11‑72: Market Value by Country, Colonic Dilation Balloon Market, Europe, 2018 – 2028 (US$M) |
| Figure 11‑73: Leading Competitors, Stenting and Dilation Device Market, Europe, 2021 |
| Figure 11‑74: Leading Competitors by Country, Stenting and Dilation Device Market, Europe, 2021 |
| Figure 12‑1: Endoscopic Retrograde Cholangiopancreatography Device Market by Segment, Europe, 2018 – 2028 (€) (1 of 2) |
| Figure 12‑2: Endoscopic Retrograde Cholangiopancreatography Device Market by Segment, Europe, 2018 – 2028 (€) (2 of 2) |
| Figure 12‑3: Endoscopic Retrograde Cholangiopancreatography Device Market by Segment, Europe, 2018 – 2028 (US$M) (1 of 2) |
| Figure 12‑4: Endoscopic Retrograde Cholangiopancreatography Device Market by Segment, Europe, 2018 – 2028 (US$M) (2 of 2) |
| Figure 12‑5: Sphincterotome Market, Europe, 2018 – 2028 |
| Figure 12‑6: Units Sold by Country, Sphincterotome Market, Europe, 2018 – 2028 |
| Figure 12‑7: Average Selling Price by Country, Sphincterotome Market, Europe, 2018 – 2028 (€) |
| Figure 12‑8: Average Selling Price by Country, Sphincterotome Market, Europe, 2018 – 2028 (US$) |
| Figure 12‑9: Market Value by Country, Sphincterotome Market, Europe, 2018 – 2028 (€M) |
| Figure 12‑10: Market Value by Country, Sphincterotome Market, Europe, 2018 – 2028 (US$M) |
| Figure 12‑11: Biliary Stone Removal Balloon Market, Europe, 2018 – 2028 |
| Figure 12‑12: Units Sold by Country, Biliary Stone Removal Balloon Market, Europe, 2018 – 2028 |
| Figure 12‑13: Average Selling Price by Country, Biliary Stone Removal Balloon Market, Europe, 2018 – 2028 (€) |
| Figure 12‑14: Average Selling Price by Country, Biliary Stone Removal Balloon Market, Europe, 2018 – 2028 (US$) |
| Figure 12‑15: Market Value by Country, Biliary Stone Removal Balloon Market, Europe, 2018 – 2028 (€M) |
| Figure 12‑16: Market Value by Country, Biliary Stone Removal Balloon Market, Europe, 2018 – 2028 (US$M) |
| Figure 12‑17: Biliary Stone Removal Basket Market, Europe, 2018 – 2028 |
| Figure 12‑18: Units Sold by Country, Biliary Stone Removal Basket Market, Europe, 2018 – 2028 |
| Figure 12‑19: Average Selling Price by Country, Biliary Stone Removal Basket Market, Europe, 2018 – 2028 (€) |
| Figure 12‑20: Average Selling Price by Country, Biliary Stone Removal Basket Market, Europe, 2018 – 2028 (US$) |
| Figure 12‑21: Market Value by Country, Biliary Stone Removal Basket Market, Europe, 2018 – 2028 (€M) |
| Figure 12‑22: Market Value by Country, Biliary Stone Removal Basket Market, Europe, 2018 – 2028 (US$M) |
| Figure 12‑23: Biliary Dilation Balloon Market, Europe, 2018 – 2028 |
| Figure 12‑24: Units Sold by Country, Biliary Dilation Balloon Market, Europe, 2018 – 2028 |
| Figure 12‑25: Average Selling Price by Country, Biliary Dilation Balloon Market, Europe, 2018 – 2028 (€) |
| Figure 12‑26: Average Selling Price by Country, Biliary Dilation Balloon Market, Europe, 2018 – 2028 (US$) |
| Figure 12‑27: Market Value by Country, Biliary Dilation Balloon Market, Europe, 2018 – 2028 (€M) |
| Figure 12‑28: Market Value by Country, Biliary Dilation Balloon Market, Europe, 2018 – 2028 (US$M) |
| Figure 12‑29: Biliary and Pancreatic Stent Market by Segment, Europe, 2018 – 2028 (€M) |
| Figure 12‑30: Biliary and Pancreatic Stent Market by Segment, Europe, 2018 – 2028 (US$M) |
| Figure 12‑31: Total Biliary and Pancreatic Stent Market, Europe, 2018 – 2028 |
| Figure 12‑32: Units Sold by Country, Total Biliary and Pancreatic Stent Market, Europe, 2018 – 2028 |
| Figure 12‑33: Average Selling Price by Country, Total Biliary and Pancreatic Stent Market, Europe, 2018 – 2028 (€) |
| Figure 12‑34: Average Selling Price by Country, Total Biliary and Pancreatic Stent Market, Europe, 2018 – 2028 (US$) |
| Figure 12‑35: Market Value by Country, Total Biliary and Pancreatic Stent Market, Europe, 2018 – 2028 (€M) |
| Figure 12‑36: Market Value by Country, Total Biliary and Pancreatic Stent Market, Europe, 2018 – 2028 (US$M) |
| Figure 12‑37: Biliary and Pancreatic Covered-Metal Stent Market by Segment, Europe, 2018 – 2028 (€M) |
| Figure 12‑38: Biliary and Pancreatic Covered-Metal Stent Market by Segment, Europe, 2018 – 2028 (US$M) |
| Figure 12‑39: Biliary and Pancreatic Covered-Metal Stent Market, Europe, 2018 – 2028 |
| Figure 12‑40: Units Sold by Country, Biliary and Pancreatic Covered-Metal Stent Market, Europe, 2018 – 2028 |
| Figure 12‑41: Average Selling Price by Country, Biliary and Pancreatic Covered-Metal Stent Market, Europe, 2018 – 2028 (€) |
| Figure 12‑42: Average Selling Price by Country, Biliary and Pancreatic Covered-Metal Stent Market, Europe, 2018 – 2028 (US$) |
| Figure 12‑43: Market Value by Country, Biliary and Pancreatic Covered-Metal Stent Market, Europe, 2018 – 2028 (€M) |
| Figure 12‑44: Market Value by Country, Biliary and Pancreatic Covered-Metal Stent Market, Europe, 2018 – 2028 (US$M) |
| Figure 12‑45: Standard Covered-Metal Stent Market, Europe, 2018 – 2028 |
| Figure 12‑46: Units Sold by Country, Standard Covered-Metal Stent Market, Europe, 2018 – 2028 |
| Figure 12‑47: Average Selling Price by Country, Standard Covered-Metal Stent Market, Europe, 2018 – 2028 (€) |
| Figure 12‑48: Average Selling Price by Country, Standard Covered-Metal Stent Market, Europe, 2018 – 2028 (US$) |
| Figure 12‑49: Market Value by Country, Standard Covered-Metal Stent Market, Europe, 2018 – 2028 (€M) |
| Figure 12‑50: Market Value by Country, Standard Covered-Metal Stent Market, Europe, 2018 – 2028 (US$M) |
| Figure 12‑51: Lumen-Apposing Covered-Metal Stent Market, Europe, 2018 – 2028 |
| Figure 12‑52: Units Sold by Country, Lumen-Apposing Covered-Metal Stent Market, Europe, 2018 – 2028 |
| Figure 12‑53: Average Selling Price by Country, Lumen-Apposing Covered-Metal Stent Market, Europe, 2018 – 2028 (€) |
| Figure 12‑54: Average Selling Price by Country, Lumen-Apposing Covered-Metal Stent Market, Europe, 2018 – 2028 (US$) |
| Figure 12‑55: Market Value by Country, Lumen-Apposing Covered-Metal Stent Market, Europe, 2018 – 2028 (€M) |
| Figure 12‑56: Market Value by Country, Lumen-Apposing Covered-Metal Stent Market, Europe, 2018 – 2028 (US$M) |
| Figure 12‑57: Biliary and Pancreatic Bare-Metal Stent Market, Europe, 2018 – 2028 |
| Figure 12‑58: Units Sold by Country, Biliary and Pancreatic Bare-Metal Stent Market, Europe, 2018 – 2028 |
| Figure 12‑59: Average Selling Price by Country, Biliary and Pancreatic Bare-Metal Stent Market, Europe, 2018 – 2028 (€) |
| Figure 12‑60: Average Selling Price by Country, Biliary and Pancreatic Bare-Metal Stent Market, Europe, 2018 – 2028 (US$) |
| Figure 12‑61: Market Value by Country, Biliary and Pancreatic Bare-Metal Stent Market, Europe, 2018 – 2028 (€M) |
| Figure 12‑62: Market Value by Country, Biliary and Pancreatic Bare-Metal Stent Market, Europe, 2018 – 2028 (US$M) |
| Figure 12‑63: Biliary and Pancreatic Plastic Stent Market, Europe, 2018 – 2028 |
| Figure 12‑64: Units Sold by Country, Biliary and Pancreatic Plastic Stent Market, Europe, 2018 – 2028 |
| Figure 12‑65: Average Selling Price by Country, Biliary and Pancreatic Plastic Stent Market, Europe, 2018 – 2028 (€) |
| Figure 12‑66: Average Selling Price by Country, Biliary and Pancreatic Plastic Stent Market, Europe, 2018 – 2028 (US$) |
| Figure 12‑67: Market Value by Country, Biliary and Pancreatic Plastic Stent Market, Europe, 2018 – 2028 (€M) |
| Figure 12‑68: Market Value by Country, Biliary and Pancreatic Plastic Stent Market, Europe, 2018 – 2028 (US$M) |
| Figure 12‑69: Biliary Lithotripter Market, Europe, 2018 – 2028 |
| Figure 12‑70: Units Sold by Country, Biliary Lithotripter Market, Europe, 2018 – 2028 |
| Figure 12‑71: Average Selling Price by Country, Biliary Lithotripter Market, Europe, 2018 – 2028 (€) |
| Figure 12‑72: Average Selling Price by Country, Biliary Lithotripter Market, Europe, 2018 – 2028 (US$) |
| Figure 12‑73: Market Value by Country, Biliary Lithotripter Market, Europe, 2018 – 2028 (€M) |
| Figure 12‑74: Market Value by Country, Biliary Lithotripter Market, Europe, 2018 – 2028 (US$M) |
| Figure 12‑75: ERCP Guidewire Market, Europe, 2018 – 2028 |
| Figure 12‑76: Units Sold by Country, ERCP Guidewire Market, Europe, 2018 – 2028 |
| Figure 12‑77: Average Selling Price by Country, ERCP Guidewire Market, Europe, 2018 – 2028 (€) |
| Figure 12‑78: Average Selling Price by Country, ERCP Guidewire Market, Europe, 2018 – 2028 (US$) |
| Figure 12‑79: Market Value by Country, ERCP Guidewire Market, Europe, 2018 – 2028 (€M) |
| Figure 12‑80: Market Value by Country, ERCP Guidewire Market, Europe, 2018 – 2028 (US$M) |
| Figure 12‑81: ERCP Cannula Market, Europe, 2018 – 2028 |
| Figure 12‑82: Units Sold by Country, ERCP Cannula Market, Europe, 2018 – 2028 |
| Figure 12‑83: Average Selling Price by Country, ERCP Cannula Market, Europe, 2018 – 2028 (€) |
| Figure 12‑84: Average Selling Price by Country, ERCP Cannula Market, Europe, 2018 – 2028 (US$) |
| Figure 12‑85: Market Value by Country, ERCP Cannula Market, Europe, 2018 – 2028 (€M) |
| Figure 12‑86: Market Value by Country, ERCP Cannula Market, Europe, 2018 – 2028 (US$M) |
| Figure 12‑87: Leading Competitors, ERCP Device Market, Europe, 2021 |
| Figure 12‑88: Leading Competitors by Country, ERCP Device Market, Europe, 2021 |
| Figure 12‑89: Leading Competitors by Country, Biliary Dilation Balloon Market, Europe, 2021 |
| Figure 12‑90: Leading Competitors by Country, Biliary and Pancreatic Stent Market, Europe, 2021 |
| Figure 12‑91: Leading Competitors by Country, Other ERCP Devices, Europe, 2021 |
| Figure 13‑1: Biopsy Forceps, Polypectomy Snare and EUS Needle Market by Segment, Europe, 2018 – 2028 (€M) |
| Figure 13‑2: Biopsy Forceps, Polypectomy Snare and EUS Needle Market by Segment, Europe, 2018 – 2028 (US$M) |
| Figure 13‑3: Biopsy Forceps Market by Segment, Europe, 2018 – 2028 (€M) |
| Figure 13‑4: Biopsy Forceps Market by Segment, Europe, 2018 – 2028 (US$M) |
| Figure 13‑5: Total Biopsy Forceps Market, Europe, 2018 – 2028 |
| Figure 13‑6: Units Sold by Country, Total Biopsy Forceps Market, Europe, 2018 – 2028 |
| Figure 13‑7: Average Selling Price by Country, Total Biopsy Forceps Market, Europe, 2018 – 2028 (€) |
| Figure 13‑8: Average Selling Price by Country, Total Biopsy Forceps Market, Europe, 2018 – 2028 (US$) |
| Figure 13‑9: Market Value by Country, Total Biopsy Forceps Market, Europe, 2018 – 2028 (€M) |
| Figure 13‑10: Market Value by Country, Total Biopsy Forceps Market, Europe, 2018 – 2028 (US$M) |
| Figure 13‑11: Cold Disposable Market, Europe, 2018 – 2028 |
| Figure 13‑12: Units Sold by Country, Cold Disposable Market, Europe, 2018 – 2028 |
| Figure 13‑13: Average Selling Price by Country, Cold Disposable Market, Europe, 2018 – 2028 (€) |
| Figure 13‑14: Average Selling Price by Country, Cold Disposable Market, Europe, 2018 – 2028 (US$) |
| Figure 13‑15: Market Value by Country, Cold Disposable Market, Europe, 2018 – 2028 (€M) |
| Figure 13‑16: Market Value by Country, Cold Disposable Market, Europe, 2018 – 2028 (US$M) |
| Figure 13‑17: Hot Disposable Market, Europe, 2018 – 2028 |
| Figure 13‑18: Units Sold by Country, Hot Disposable Market, Europe, 2018 – 2028 |
| Figure 13‑19: Average Selling Price by Country, Hot Disposable Market, Europe, 2018 – 2028 (€) |
| Figure 13‑20: Average Selling Price by Country, Hot Disposable Market, Europe, 2018 – 2028 (US$) |
| Figure 13‑21: Market Value by Country, Hot Disposable Market, Europe, 2018 – 2028 (€M) |
| Figure 13‑22: Market Value by Country, Hot Disposable Market, Europe, 2018 – 2028 (US$M) |
| Figure 13‑23: Cold Reusable Market, Europe, 2018 – 2028 |
| Figure 13‑24: Units Sold by Country, Cold Reusable Market, Europe, 2018 – 2028 |
| Figure 13‑25: Average Selling Price by Country, Cold Reusable Market, Europe, 2018 – 2028 (€) |
| Figure 13‑26: Average Selling Price by Country, Cold Reusable Market, Europe, 2018 – 2028 (US$) |
| Figure 13‑27: Market Value by Country, Cold Reusable Market, Europe, 2018 – 2028 (€M) |
| Figure 13‑28: Market Value by Country, Cold Reusable Market, Europe, 2018 – 2028 (US$M) |
| Figure 13‑29: Hot Reusable Market, Europe, 2018 – 2028 |
| Figure 13‑30: Units Sold by Country, Hot Reusable Market, Europe, 2018 – 2028 |
| Figure 13‑31: Average Selling Price by Country, Hot Reusable Market, Europe, 2018 – 2028 (€) |
| Figure 13‑32: Average Selling Price by Country, Hot Reusable Market, Europe, 2018 – 2028 (US$) |
| Figure 13‑33: Market Value by Country, Hot Reusable Market, Europe, 2018 – 2028 (€M) |
| Figure 13‑34: Market Value by Country, Hot Reusable Market, Europe, 2018 – 2028 (US$M) |
| Figure 13‑35: Polypectomy Snare Market, Europe, 2018 – 2028 |
| Figure 13‑36: Units Sold by Country, Polypectomy Snare Market, Europe, 2018 – 2028 |
| Figure 13‑37: Average Selling Price by Country, Polypectomy Snare Market, Europe, 2018 – 2028 (€) |
| Figure 13‑38: Average Selling Price by Country, Polypectomy Snare Market, Europe, 2018 – 2028 (US$) |
| Figure 13‑39: Market Value by Country, Polypectomy Snare Market, Europe, 2018 – 2028 (€M) |
| Figure 13‑40: Market Value by Country, Polypectomy Snare Market, Europe, 2018 – 2028 (US$M) |
| Figure 13‑41: EUS Needle Market, Europe, 2018 – 2028 |
| Figure 13‑42: Units Sold by Country, EUS Needle Market, Europe, 2018 – 2028 |
| Figure 13‑43: Average Selling Price by Country, EUS Needle Market, Europe, 2018 – 2028 (€) |
| Figure 13‑44: Average Selling Price by Country, EUS Needle Market, Europe, 2018 – 2028 (US$) |
| Figure 13‑45: Market Value by Country, EUS Needle Market, Europe, 2018 – 2028 (€M) |
| Figure 13‑46: Market Value by Country, EUS Needle Market, Europe, 2018 – 2028 (US$M) |
| Figure 13‑47: Leading Competitors, Biopsy Forceps, Polypectomy Snare and EUS Needle Market, Europe, 2021 |
| Figure 13‑48: Leading Competitors by Country, Biopsy Forceps, Polypectomy Snare and EUS Needle Market, Europe, 2021 |
| Figure 14‑1: Endoscopic Submucosal Dissection Knife Market, Europe, 2018 – 2028 |
| Figure 14‑2: Leading Competitors, Endoscopic Submucosal Dissection Knife Market, Europe, 2021 |
| Figure 15‑1: Hemostasis Device Market by Segment, Europe, 2018 – 2028 (€M) |
| Figure 15‑2: Hemostasis Device Market by Segment, Europe, 2018 – 2028 (US$M) |
| Figure 15‑3: Hemostasis Probe Market by Segment, Europe, 2018 – 2028 (€M) |
| Figure 15‑4: Total Hemostasis Probe Market by Segment, Europe, 2018 – 2028 (US$M) |
| Figure 15‑5: Total Hemostasis Probe Market, Europe, 2018 – 2028 |
| Figure 15‑6: Units Sold by Country, Total Hemostasis Probe Market, Europe, 2018 – 2028 |
| Figure 15‑7: Average Selling Price by Country, Total Hemostasis Probe Market, Europe, 2018 – 2028 (€) |
| Figure 15‑8: Average Selling Price by Country, Total Hemostasis Probe Market, Europe, 2018 – 2028 (US$) |
| Figure 15‑9: Market Value by Country, Total Hemostasis Probe Market, Europe, 2018 – 2028 (€M) |
| Figure 15‑10: Market Value by Country, Total Hemostasis Probe Market, Europe, 2018 – 2028 (US$M) |
| Figure 15‑11: Electrosurgical Probe Market, Europe, 2018 – 2028 |
| Figure 15‑12: Units Sold by Country, Electrosurgical Probe Market, Europe, 2018 – 2028 |
| Figure 15‑13: Average Selling Price by Country, Electrosurgical Probe Market, Europe, 2018 – 2028 (€) |
| Figure 15‑14: Average Selling Price by Country, Electrosurgical Probe Market, Europe, 2018 – 2028 (US$) |
| Figure 15‑15: Market Value by Country, Electrosurgical Probe Market, Europe, 2018 – 2028 (€M) |
| Figure 15‑16: Market Value by Country, Electrosurgical Probe Market, Europe, 2018 – 2028 (US$M) |
| Figure 15‑17: Heat Probe Market, Europe, 2018 – 2028 |
| Figure 15‑18: Units Sold by Country, Heat Probe Market, Europe, 2018 – 2028 |
| Figure 15‑19: Average Selling Price by Country, Heat Probe Market, Europe, 2018 – 2028 (€) |
| Figure 15‑20: Average Selling Price by Country, Heat Probe Market, Europe, 2018 – 2028 (US$) |
| Figure 15‑21: Market Value by Country, Heat Probe Market, Europe, 2018 – 2028 (€M) |
| Figure 15‑22: Market Value by Country, Heat Probe Market, Europe, 2018 – 2028 (US$M) |
| Figure 15‑23: Argon Probe Market, Europe, 2018 – 2028 |
| Figure 15‑24: Units Sold by Country, Argon Probe Market, Europe, 2018 – 2028 |
| Figure 15‑25: Average Selling Price by Country, Argon Probe Market, Europe, 2018 – 2028 (€) |
| Figure 15‑26: Average Selling Price by Country, Argon Probe Market, Europe, 2018 – 2028 (US$) |
| Figure 15‑27: Market Value by Country, Argon Probe Market, Europe, 2018 – 2028 (€M) |
| Figure 15‑28: Market Value by Country, Argon Probe Market, Europe, 2018 – 2028 (US$M) |
| Figure 15‑29: Ligation Device Market by Segment, Europe, 2018 – 2028 (€M) |
| Figure 15‑30: Ligation Device Market by Segment, Europe, 2018 – 2028 (US$M) |
| Figure 15‑31: Total Ligation Device Market, Europe, 2018 – 2028 |
| Figure 15‑32: Units Sold by Country, Total Ligation Device Market, Europe, 2018 – 2028 |
| Figure 15‑33: Average Selling Price by Country, Total Ligation Device Market, Europe, 2018 – 2028 (€) |
| Figure 15‑34: Average Selling Price by Country, Total Ligation Device Market, Europe, 2018 – 2028 (US$) |
| Figure 15‑35: Market Value by Country, Total Ligation Device Market, Europe, 2018 – 2028 (€M) |
| Figure 15‑36: Market Value by Country, Total Ligation Device Market, Europe, 2018 – 2028 (US$M) |
| Figure 15‑37: Ligation Band Market, Europe, 2018 – 2028 |
| Figure 15‑38: Units Sold by Country, Ligation Band Market, Europe, 2018 – 2028 |
| Figure 15‑39: Average Selling Price by Country, Ligation Band Market, Europe, 2018 – 2028 (€) |
| Figure 15‑40: Average Selling Price by Country, Ligation Band Market, Europe, 2018 – 2028 (US$) |
| Figure 15‑41: Market Value by Country, Ligation Band Market, Europe, 2018 – 2028 (€M) |
| Figure 15‑42: Market Value by Country, Ligation Band Market, Europe, 2018 – 2028 (US$M) |
| Figure 15‑43: Ligation Clip Market, Europe, 2018 – 2028 |
| Figure 15‑44: Units Sold by Country, Ligation Clip Market, Europe, 2018 – 2028 |
| Figure 15‑45: Average Selling Price by Country, Ligation Clip Market, Europe, 2018 – 2028 (€) |
| Figure 15‑46: Average Selling Price by Country, Ligation Clip Market, Europe, 2018 – 2028 (US$) |
| Figure 15‑47: Market Value by Country, Ligation Clip Market, Europe, 2018 – 2028 (€M) |
| Figure 15‑48: Market Value by Country, Ligation Clip Market, Europe, 2018 – 2028 (US$M) |
| Figure 15‑49: Sclerotherapy Needle Market, Europe, 2018 – 2028 |
| Figure 15‑50: Units Sold by Country, Sclerotherapy Needle Market, Europe, 2018 – 2028 |
| Figure 15‑51: Average Selling Price by Country, Sclerotherapy Needle Market, Europe, 2018 – 2028 (€) |
| Figure 15‑52: Average Selling Price by Country, Sclerotherapy Needle Market, Europe, 2018 – 2028 (US$) |
| Figure 15‑53: Market Value by Country, Sclerotherapy Needle Market, Europe, 2018 – 2028 (€M) |
| Figure 15‑54: Market Value by Country, Sclerotherapy Needle Market, Europe, 2018 – 2028 (US$M) |
| Figure 15‑55: Leading Competitors by Country, Hemostasis Device Market, Europe, 2021 |
| Figure 16‑1: Enteral Feeding Device Market by Segment, Europe, 2018 – 2028 (€M) |
| Figure 16‑2: Enteral Feeding Device Market by Segment, Europe, 2018 – 2028 (US$M) |
| Figure 16‑3: Total Enteral Feeding Device Market, Europe, 2018 – 2028 |
| Figure 16‑4: Units Sold by Country, Total Enteral Feeding Tube Market, Europe, 2018 – 2028 |
| Figure 16‑5: Average Selling Price by Country, Total Enteral Feeding Tube Market, Europe, 2018 – 2028 (€) |
| Figure 16‑6: Average Selling Price by Country, Total Enteral Feeding Tube Market, Europe, 2018 – 2028 (US$) |
| Figure 16‑7: Market Value by Country, Total Enteral Feeding Tube Market, Europe, 2018 – 2028 (€M) |
| Figure 16‑8: Market Value by Country, Total Enteral Feeding Tube Market, Europe, 2018 – 2028 (US$M) |
| Figure 16‑9: PEG Tube Market by Segment, Europe, 2018 – 2028(€M) |
| Figure 16‑10: PEG Tube Market by Segment, Europe, 2018 – 2028(US$M) |
| Figure 16‑11: Total PEG Tube Market, Europe, 2018 – 2028 |
| Figure 16‑12: Units Sold by Country, PEG Tube Market, Europe, 2018 – 2028 |
| Figure 16‑13: Average Selling Price by Country, PEG Tube Market, Europe, 2018 – 2028 (€) |
| Figure 16‑14: Average Selling Price by Country, PEG Tube Market, Europe, 2018 – 2028 (US$) |
| Figure 16‑15: Market Value by Country, PEG Tube Market, Europe, 2018 – 2028 (€M) |
| Figure 16‑16: Market Value by Country, PEG Tube Market, Europe, 2018 – 2028 (US$M) |
| Figure 16‑17: Initial Placement PEG Tube Market, Europe, 2018 – 2028 |
| Figure 16‑18: Units Sold by Country, Initial Placement PEG Tube Market, Europe, 2018 – 2028 |
| Figure 16‑19: Average Selling Price by Country, Initial Placement PEG Tube Market, Europe, 2018 – 2028 (€) |
| Figure 16‑20: Average Selling Price by Country, Initial Placement PEG Tube Market, Europe, 2018 – 2028 (US$) |
| Figure 16‑21: Market Value by Country, Initial Placement PEG Tube Market, Europe, 2018 – 2028 (€M) |
| Figure 16‑22: Market Value by Country, Initial Placement PEG Tube Market, Europe, 2018 – 2028 (US$M) |
| Figure 16‑23: Replacement PEG Tube Market, Europe, 2018 – 2028 |
| Figure 16‑24: Units Sold by Country, Replacement PEG Tube Market, Europe, 2018 – 2028 |
| Figure 16‑25: Average Selling Price by Country, Replacement PEG Tube Market, Europe, 2018 – 2028 (€) |
| Figure 16‑26: Average Selling Price by Country, Replacement PEG Tube Market, Europe, 2018 – 2028 (US$) |
| Figure 16‑27: Market Value by Country, Replacement PEG Tube Market, Europe, 2018 – 2028 (€M) |
| Figure 16‑28: Market Value by Country, Replacement PEG Tube Market, Europe, 2018 – 2028 (US$M) |
| Figure 16‑29: NG/NJ Tube Market, Europe, 2018 – 2028 |
| Figure 16‑30: Units Sold by Country, NG/NJ Tube Market, Europe, 2018 – 2028 |
| Figure 16‑31: Average Selling Price by Country, NG/NJ Tube Market, Europe, 2018 – 2028 (€) |
| Figure 16‑32: Average Selling Price by Country, NG/NJ Tube Market, Europe, 2018 – 2028 (US$) |
| Figure 16‑33: Market Value by Country, NG/NJ Tube Market, Europe, 2018 – 2028 (€M) |
| Figure 16‑34: Market Value by Country, NG/NJ Tube Market, Europe, 2018 – 2028 (US$M) |
| Figure 16‑35: Leading Competitors by Country, Enteral Feeding Device Market, Europe, 2021 |
| Figure 17‑1: Anti-Reflux Device Market, Europe, 2018 – 2028 |
| Figure 17‑2: Leading Competitors, Anti-Reflux Device Market, Europe, 2021 |

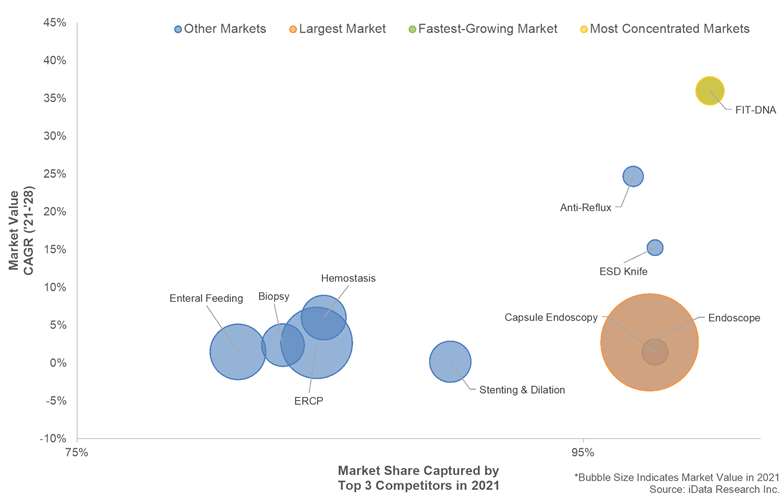
 Olympus held the leading share of both the gastrointestinal (GI) endoscope market and endoscopic submucosal dissection (ESD) knife market, as well as competitive shares of the capsule endoscopy, ERCP, biopsy forceps, polypectomy snares and EUS needles and hemostasis markets.
Olympus held the leading share of both the gastrointestinal (GI) endoscope market and endoscopic submucosal dissection (ESD) knife market, as well as competitive shares of the capsule endoscopy, ERCP, biopsy forceps, polypectomy snares and EUS needles and hemostasis markets.