 Register to receive a free U.S. Market Report Suite for Peripheral Vascular Devices and Accessories – MedSuite report synopsis and brochure
Register to receive a free U.S. Market Report Suite for Peripheral Vascular Devices and Accessories – MedSuite report synopsis and brochure
The U.S. Food and Drug Administration has released a warning letter to peripheral interventionalists and vascular medicine physicians regarding an increase in deaths involving paclitaxel-coated balloons and stents in a recent study.
The notice outlines the heightened mortality risk at 2 years postoperative in a late meta-analysis of peripheral artery disease (PAD) from the Journal of the American Heart Association concerning patients treated with paclitaxel-coated devices when compared to patients treated with non-coated balloons or bare-metal stents.
As such, the drug-coated balloon (DCB) market, once an engine of MedTech growth, was dealt another blow this month as the FDA escalated these concerns of increased 2-year mortality rates in patients treated with Paclitaxel-coated devices. Medtronic will likely be the hardest hit by these revelations as its IN.PACT™ Admiral™ DCB features a higher drug dose than comparable products from Becton Dickinson (Lutonix®) and Philips (Stellarex™).
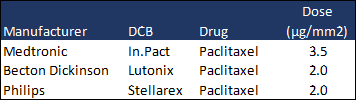
Source: iData Research
This comes just one year after substantial reimbursement cuts in the DCB market following the expiry of the CMS’s transitional pass-through (TPT) payment. The reduced payment has caused many end-users and purchasers to re-evaluate the value of DCBs, driving modest price declines across the U.S. market.
However, the FDA states that the benefits of approved paclitaxed-coated devices still outweigh their risks when implemented following their indications of use. The agency emphasizes the importance of consistently monitoring patients with these devices and apt discussion of the risks and benefits of all available treatment options between the physician and patient.
The agency will be analyzing long-term follow-up data on these devices to investigate further, including studies that supported these products’ initial FDA approval.
For Further Information
More on the peripheral vascular stent market in the United States can be found in a report series published by iData Research entitled the U.S. Peripheral Vascular Devices Market.
Reports provide a comprehensive analysis including units sold, procedure numbers, market value, forecasts, as well as detailed competitive market shares and analysis of major players’ success strategies in each market and segment. To find out more about peripheral vascular device market data or procedure data, register online or email us at info@idataresearch.net for a U.S. Peripheral Vascular Devices brochure and synopsis.
 Register to receive a free
Register to receive a free