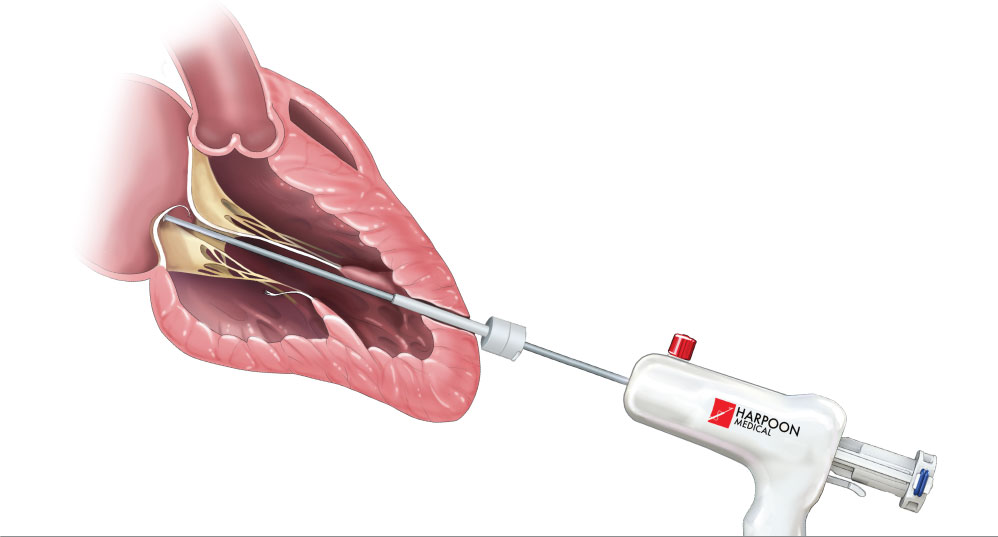
 The Harpoon™ Beating Heart Mitral Valve System
The Harpoon™ Beating Heart Mitral Valve System
When the mitral valve prolapses, blood leaks back into the atrium each time the heart beats. This condition, aptly named degenerative mitral regurgitation (DMR), can be asymptomatic for many patients, but for some it may cause fainting, pain, shortness of breath, and more. According to the University of Maryland, In a year, less than 2 percent of patients with severe mitral regurgitation receive surgical treatment. Low treatments rates could be attributed to the referrer being unaware of minimally invasive options. Due to this, surgeons are often seeking to improve the outcomes of mitral repair with simplicity, reproducibility, and reliable results.
Within the realm of modern medicine, although highly invasive, open-heart surgery is considered to be the standard treatment for degenerative mitral regurgitation. However, as we progress into modern medicine, The HARPOON™ system developed by Edwards Lifesciences is designed to provide mitral valve repair through a minimally invasive chest incision, allowing the procedure to occur while the heart is still beating. This technology is one of the first introductions of reproducible, beating-heart, off-pump surgical mitral repair.
Based on familiar surgical principles, the HARPOON™ system utilized small-footprint ePTFE chords with proprietary self-forming knots that are easily deployed and anchored using the preloaded HARPOON™ delivery system. With real-time choral adjustment on the beating heart, the practitioner is capable of ensuring optimal coaptation and overall reduction of MR.
The HARPOON™ system is dedicated to re-envisioning the surgical experience whilst improving overall patient experience. Results from a prospective European study have demonstrated the following benefits of the HARPOON™ system:
- Least Invasive Surgical Approach
- Performed on a Beating Heart
- Less Blood Loss
- Shorter Operation Times
- Simplification of MV Repair
According the the University of Maryland Heart & Vascular Center’s Critical Mass of Mitral and Tricuspid Valve Trials, in an attempt to receive FDA approval, the HARPOON™ device is currently under investigation by the U.S. centers in a non-randomized safety and efficacy trial: Beating Heart Mitral Valve Repair with the HARPOON™™ System (RESTORE). The trial is open at varying centers, including the University of Maryland. Studies of the HARPOON™ device are being funded by Edwards Lifesciences. As of current, the U.S. Food and Drug Administration and CE have not approved the HARPOON™ device for patients in the U.S. or Europe.
For more information on the U.S. Cardiac Surgery and Heart Valve Devices Market, including unit sales, average selling prices, market drivers and limiters, competitive market share analysis, and more, follow the link below to receive a free research summary of this report. In addition, we offer our complete Global Cardiac Surgery Market report series which includes the analysis of the endoscopy market in 70 countries and 7 regions.