 Register to receive a free U.S. Market Report Suite for Peripheral Vascular Devices and Accessories – MedSuite report synopsis and brochure
Register to receive a free U.S. Market Report Suite for Peripheral Vascular Devices and Accessories – MedSuite report synopsis and brochure
Contego Medical, LLC has announced the beginning of enrollment in the PERFORMANCE I Trial, a clinical trial designed to evaluate the safety and feasibility of the Neuroguard IEP® 3-in-1 Carotid Stent and Post-Dilation Balloon System with Integrated Embolic Protection. The Neuroguard IEP System is designed to treat clinically significant carotid artery stenosis, further expanding Contego Medical’s portfolio of neurovascular solutions.
Prof. Saško Kedev, M.D., Ph.D., of University Clinic of Cardiology in Skopje, Macedonia treated the first patient enrolled in the PERFORMANCE I Trial. “We are pleased to initiate this trial evaluating the Neuroguard IEP System, the first of its kind for carotid stenting,” remarked Prof. Kedev. “Protection from stroke is key during carotid artery interventions. This 3-in-1 system includes a novel state-of-the-art carotid stent and provides uninterrupted micro-embolic protection during the most vulnerable stages of the procedure. I am very impressed with the performance of all aspects of the device.”
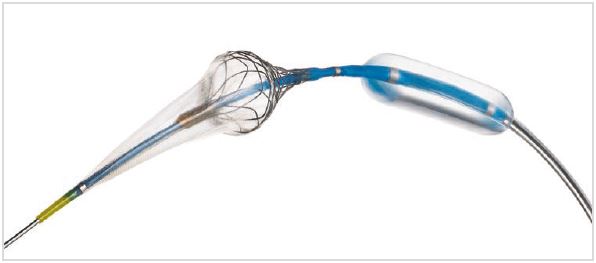
The Neuroguard IEP System contains a novel, next-generation nitinol stent, a pre-positioned post-dilation balloon and an integrated microembolic filter with 40 μm pores. The stent has a closed-cell design for optimal balance of radial strength and flexibility. The stent is flared at both ends to facilitate ideal wall apposition in tortuous anatomy. The integrated filter is designed to capture both macro- and micro-emboli during the entire intervention. All components are mounted on a 6 French delivery system.
“The Neuroguard IEP System signals a new era in carotid artery stenting,” said Ravish Sachar, M.D., CEO and Founder of Contego Medical. “By combining a novel stent, post-dilation balloon, and embolic filter onto the same catheter, we expect to dramatically impact this procedure by improving ease of use, reducing interventional time, and maximizing embolic protection. The Neuroguard IEP System represents Contego’s third innovation in our commitment to deliver endovascular devices designed to improve outcomes without increasing complexity.”
About Contego Medical
Contego Medical, LLC is a medical device company dedicated to the development of novel medical devices for cardiovascular and peripheral vascular procedures. The company’s Integrated Embolic Protection (IEP) platform, which combines embolic protection and treatment into one device, is designed to simplify catheter-based procedures and improve patient outcomes.
The Neuroguard IEP System is an investigational device and not commercially available, worldwide.
For Further Information
More on the peripheral vascular stent market in the U.S. can be found in a report series published by iData Research entitled the U.S. Market Report Suite for Peripheral Vascular Devices and Accessories. The suite covers reports on the following markets: Peripheral Vascular Stent Market, PTA Balloon Catheter Market, Atherectomy Device Market, Chronic Total Occlusion (CTO) Device Market, Embolic Protection Device Market, Stent Graft Market, Surgical Graft Market, Diagnostic and Interventional Catheter Market, Diagnostic and Interventional Guidewire Market, Introducer Sheath Market, Inferior Vena Cava Filter Market, Arteriovenous (AV) Access Thrombectomy Device Market, Vascular Closure Device Market, and Transcatheter Embolization Device Market.
Reports also provide a comprehensive analysis including units sold, procedure numbers, market value, forecasts, as well as detailed competitive market shares and analysis of major players’ success strategies in each market and segment. To find out more about peripheral vascular device market data or procedure data, register online or email us at info@idataresearch.net for a U.S. Market Report Suite for Peripheral Vascular Devices and Accessories brochure and synopsis.
 Register to receive a free
Register to receive a free