Register to receive a free Interventional Cardiology Devices Market Report Suite for U.S. synopsis and brochure
BIOTRONIK has announced the recent U.S. Food and Drug Administration (FDA) approval of their Orsiro stent for the treatment of coronary artery disease. The product gained CE mark approval in 2011 and has already treated over a million cardiovascular patients worldwide. Orsiro is a drug-eluting stent and is the first and only ultrathin product of its kind to surpass the clinical standard, Xience.
Orsiro’s prowess was demonstrated in the BIOFLOW-V pivotal trial where it exhibited noticeably improved rates of target vessel myocardial infarction (MI) and target lesion failure (TLF) at one year when compared to Xience. Additionally, the trial encompassed a vast and complex patient population. These trends were even further improved at the two-year mark, with an additional reduction in target lesion revascularization (TLR) and spontaneous MI. In total, the two-year clinical results for the Orsiro demonstrate a 37% lower TLF rate, a 47% lower ischemia-driven TLR rate, and a 70% lower spontaneous MI rate.
“Orsiro has set a new standard for safety and efficacy clinical endpoints, including statistically lower target lesion revascularization and target vessel MI rates,” said Dr. David Kandzari, BIOFLOW-V US principal investigator, Piedmont Heart Institute, Atlanta. “BIOFLOW-V data are the best clinical outcomes witnessed with modern DES. It was largely thought that efficacy findings were unsurpassable, but Orsiro proves we can further reduce event rates with meaningful innovation.”
iData’s Interventional Cardiology market research finds that the drug-eluting stent segment dominates the total U.S. coronary stent market, with its market share just shy of 95%. The growth of unit sales in this segment will be driven by the overall growth of percutaneous coronary intervention (PCI) procedures. Significant ASP depreciation is expected due to bundling and competitive pricing, but this segment will maintain its powerful lead for years to come.
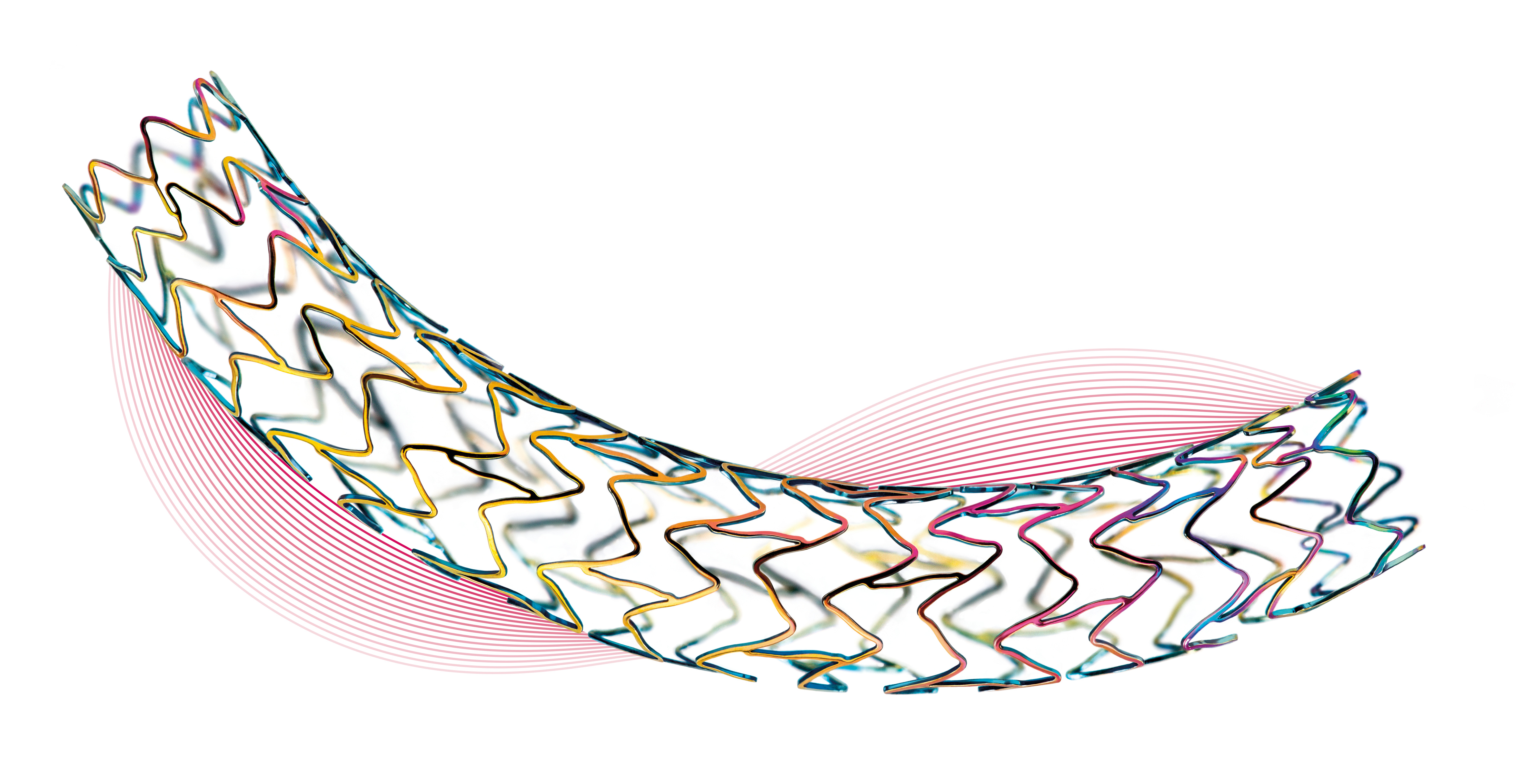
Orsiro is designed for use in PCI procedures, where the cobalt chromium metal stent elutes sirolimus via BIOTRONIK’s bioresorbable polymer coating, dubbed BIOlute™. Beneath this layer is a passive coating of BIOTRONIK’s proBIO™, which is designed to reduce nickel iron release. Orsiro boasts ultrathin stent struts while maintaining radial strength and a low crossing profile to facilitate lesion cross in more complex PCI procedures. The product is already available in the U.S., with 52 sizes ranging from 2.25 to 4.0mm in diameter and lengths up to 40mm, the longest in the United States.
“The FDA approval of Orsiro changes the dynamic of what had become a highly commoditized DES market,” said Ryan Walters, President at BIOTRONIK, Inc. “We designed Orsiro for use even in challenging cases with features that make it unlike any other DES in the world. Hospital administrators now have available a DES that shows improved clinical event rates and interventionalists can rely on Orsiro’s deliverability to treat complex lesions and challenging subgroups to achieve unprecedented patient outcomes. Patients, physicians and health systems deserve the best, and that is exactly what we are bringing to the US market.”
For Further Information
More on the interventional cardiology market in the U.S. can be found in a series of reports published by iData entitled the U.S. Market Report Suite for Interventional Cardiology Devices.