Register to receive a free European Market Report Suite for Peripheral Vascular Devices report synopsis and brochure
Avinger, Inc., a leading developer of innovative treatments for Peripheral Artery Disease (PAD), recently announced Conformité Européenne (CE) Marking approval for treating in-stent restenosis with the Pantheris® Lumivascular atherectomy system.
In-stent restenosis occurs when a previously blocked artery treated with a stent becomes narrowed again, blocking blood flow. Physicians often face challenges when treating in-stent restenosis both in terms of safety and efficacy. From a safety standpoint, limitations in imaging techniques such as X-ray fluoroscopy and the inability to control the directionality of other treatment modalities’ mechanism of action creates the concern of potentially impacting the integrity of the stent during the intervention. In terms of efficacy, current therapies for in-stent restenosis such as balloon angioplasty suffer from high rates of recurrent renarrowing within stents.
“The demand for improved treatment options for in-stent restenosis is growing as physicians experience the accurate visualization and precision provided by state-of-the-art technologies such as the Pantheris Lumivascular atherectomy system,” said Jeff Soinski, Avinger’s president and CEO. “CE Marking for this particular indication is an important milestone for Avinger that addresses an area of unmet clinical need for patients suffering from PAD. Onboard image guidance coupled with directional plaque excision offers the interventionist clear benefits when treating in-stent restenosis and represents another opportunity to improve patient outcomes.”
“Two elements thoughtful interventionists want to avoid during intervention are adventitia and stent struts,” said John B. Simpson, Ph.D., M.D., founder and executive chairman. “Intravascular visualization combined with a directional mechanism in real time provides operators the information and precision needed to treat only diseased tissue without coming into contact with the stent struts or adventitia.”
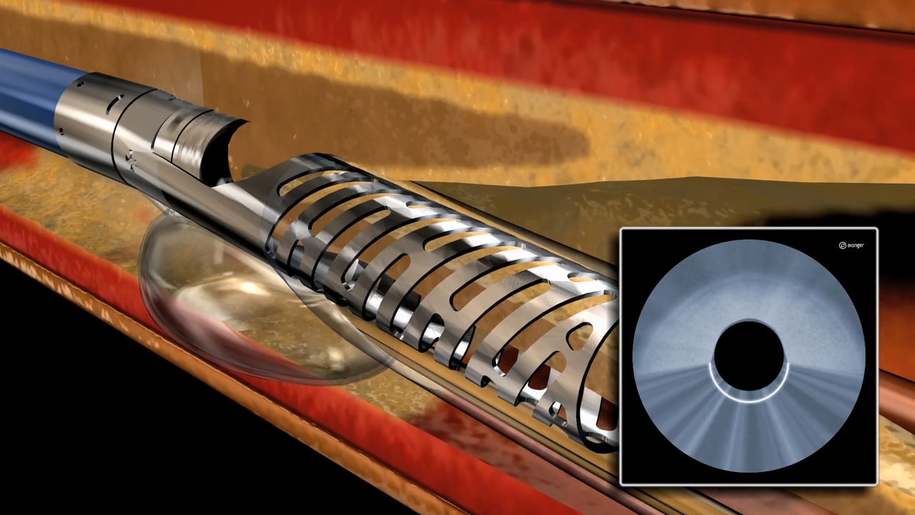
Atherectomy is a minimally invasive treatment for PAD in which a catheter-based device is used to remove plaque from a blood vessel. Lumivascular technology allows physicians, for the first time ever, to see from inside the artery during a directional atherectomy procedure by using an imaging modality called optical coherence tomography, or OCT, that is displayed on the Lightbox console. In the past, physicians have had to rely solely on X-ray as well as tactile feedback to guide their tools while treating complicated arterial disease. With the Lumivascular approach using real-time OCT image guidance, physicians can more accurately navigate their devices to treat PAD without exposing healthcare workers and patients to the negative effects of ionizing radiation.
For Further Information
More on the peripheral vascular device market in Europe can be found in a series of reports published by iData Research entitled the European Market Report Suite for Peripheral Vascular Devices. The suite covers reports on the following markets: Peripheral Vascular Stents, Percutaneous Transluminal Angioplasty Balloon Catheters, Atherectomy Devices, Chronic Total Occlusion Devices, Embolic Protection Devices, Stent-Grafts, Surgical Grafts, Arteriovenous Access Thrombectomy Devices, Inferior Vena Cava Filters, Diagnostic and Interventional Catheters, Standard and Hydrophilic Guidewires, Introducer Sheaths, Vascular Closure Devices and Transcatheter Embolization Devices.
The iData report series on peripheral vascular devices covers the U.S. and 15 countries in Europe including Austria, Belgium, Denmark, Finland, France, Germany, Italy, Luxembourg, Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, and the United Kingdom. Reports provide a comprehensive analysis including units sold, procedure numbers, market value, forecasts, as well as detailed competitive market shares and analysis of major players’ success strategies in each market and segment. To find out more about peripheral vascular market data or procedure data, register online or email us at info@idataresearch.net for a European Market Report Suite for Peripheral Vascular Devices brochure and synopsis.