| Figure 1-1: Patient Monitoring Equipment Market Share Ranking by Segment, Global, 2021 (1 of 2) 4 |
| Figure 1-2: Patient Monitoring Equipment Market Share Ranking by Segment, Global, 2021 (2 of 2) 4 |
| Figure 1-3: Companies Researched in this Report (1 of 2) 5 |
| Figure 1-4: Companies Researched in this Report (1 of 2) 6 |
| Figure 1-5: Factors Impacting the Patient Monitoring Equipment Market by Segment, Global (1 of 2) 7 |
| Figure 1-6: Factors Impacting the Patient Monitoring Equipment Market by Segment, Global (2 of 2) 8 |
| Figure 1-7: Recent Events in the Patient Monitoring Equipment Market, Global, 2018 – 2021 (1 of 2) 9 |
| Figure 1-8: Recent Events in the Patient Monitoring Equipment Market, Global, 2018 – 2021 (2 of 2) 10 |
| Figure 1-9: Patient Monitoring Equipment Markets Covered 11 |
| Figure 1-10: Patient Monitoring Equipment Regions Covered, Global (1 of 2) 12 |
| Figure 1-11: Patient Monitoring Equipment Regions Covered, Global (2 of 2) 13 |
| Figure 1-12: Version History 13 |
| Figure 2-1: Patient Monitoring Equipment Market by Segment, Worst Case Scenario, Global, 2018 – 2028 |
| (US$M) (1 of 2) 31 |
| Figure 2-2: Patient Monitoring Equipment Market by Segment, Worst Case Scenario, Global, 2018 – 2028 |
| (US$M) (2 of 2) 32 |
| Figure 2-3: Patient Monitoring Equipment Market by Segment, Base Case Scenario, Global, 2018 – 2028 |
| (US$M) (1 of 2) 33 |
| Figure 2-4: Patient Monitoring Equipment Market by Segment, Base Case Scenario, Global, 2018 – 2028 |
| (US$M) (2 of 2) 34 |
| Figure 2-5: Patient Monitoring Equipment Market by Segment, Best Case Scenario, Global, 2018 – 2028 |
| (US$M) (1 of 2) 35 |
| Figure 2-6: Patient Monitoring Equipment Market by Segment, Best Case Scenario, Global, 2018 – 2028 |
| (US$M) (2 of 2) 36 |
| Figure 3-1: Blood Pressure Monitoring Products by Company (1 of 2) 41 |
| Figure 3-2: Blood Pressure Monitoring Products by Company (2 of 2) 42 |
| Figure 3-3: Cardiac Output Monitoring Products by Company (1 of 2) 44 |
| Figure 3-4: Cardiac Output Monitoring Products by Company (2 of 2) 45 |
| Figure 3-5: Cerebral Oximetry Monitoring Products by Company 46 |
| Figure 3-6: Electroencephalogram Monitoring (EEG) Products by Company 48 |
| Figure 3-7: Electromyogram Monitoring Products by Company 50 |
| Figure 3-8: Fetal and Neonatal Monitoring Products by Company 52 |
| Figure 3-9: Intracranial Pressure Monitoring (ICP) Products by Company 53 |
| Figure 3-10: Multi-Parameter Vital Signs Monitoring Products by Company (1 of 2) 56 |
| Figure 3-11: Multi-Parameter Vital Signs Monitoring Products by Company (2 of 2) 57 |
| Figure 3-12: Pulse Oximetry Monitoring Products by Company 59 |
| Figure 3-13: Telehealth Products by Company 61 |
| Figure 3-14: Wireless Ambulatory Telemetry Monitoring Products by Company (1 of 2) 64 |
| Figure 3-15: Wireless Ambulatory Telemetry Monitoring Products by Company (2 of 2) 65 |
| Figure 3-16: Electrocardiogram Monitoring (ECG) Products by Company 67 |
| Figure 3-17: Class 2 Device Recall LATITUDE 68 |
| Figure 3-18: Class 2 Device Recall LATITUDE Programming System 68 |
| Figure 3-19: Class 2 Device Recall LATITUDE Programming System 68 |
| Figure 3-20: Class 1 Device Recall EV1000 Clinical Platform 69 |
| Figure 3-21: Class 2 Device Recall HemoSphere Advanced Monitor, HemoSphere SwanGanz Module, and |
| HemoSphere Oximetry Cable 69 |
| Figure 3-22: Class 2 Device Recall CARESCAPE ONE 70 |
| Figure 3-23: Class 2 Device Recall CARESCAPE Patient Data Module 70 |
| Figure 3-24: Class 2 Device Recall CARESCAPE Central Station 70 |
| Figure 3-25: Class 2 Device Recall B105/125 Patient Monitor 71 |
| Figure 3-26: Class 2 Device Recall Solar 8000M Patient Monitoring System 71 |
| Figure 3-27: Class 2 Device Recall GE Healthcare CARESCAPE Monitor B850 71 |
| Figure 3-28: Class 2 Device Recall GE Healthcare CARESCAPE Monitor B650 72 |
| Figure 3-29: Class 2 Device Recall Cogent Hemodynamic Monitoring System 72 |
| Figure 3-30: Class 2 Device Recall Cogent" Hemodynamic Monitoring System 72 |
| Figure 3-31: Class 2 Device Recall CareLink 2090 programmer system 73 |
| Figure 3-32: Class 1 Device Recall SENSIA, Dual chamber pacemaker 73 |
| Figure 3-33: Class 2 Device Recall MyCareLink Smart Patient Monitor 74 |
| Figure 3-34: Class 2 Device Recall DLP Pressure Disposable Pressure Display Sets 74 |
| Figure 3-35: Class 2 Device Recall Rosebud Vital Signs Monitor 75 |
| Figure 3-36: Class 2 Device Recall Mindray BeneVision Distributed Monitoring System (DMS) 75 |
| Figure 3-37: Class 2 Device Recall T1 Patient Monitor 75 |
| Figure 3-38: Class 2 Device Recall CSM1901 76 |
| Figure 3-39: Class 2 Device Recall Nonin 76 |
| Figure 3-40: Class 2 Device Recall IntelliVue MX800 77 |
| Figure 3-41: Class 2 Device Recall Philips SureSigns 77 |
| Figure 3-42: Class 2 Device Recall Philips IntelliSpace PACS 4.4 77 |
| Figure 3-43: Class 2 Device Recall NonMagnetic Chest Pneumograph Toshiba KSB 78 |
| Figure 3-44: Class 2 Device Recall Expression MR400 MRI Patient Monitoring System 78 |
| Figure 3-45: Class 2 Device Recall IntelliVue MX40 Patient Monitor 78 |
| Figure 3-46: Class 2 Device Recall SureSigns VS3 NBP 79 |
| Figure 3-47: Class 2 Device Recall Spacelabs Healthcare Xhibit Central Station (96102) 79 |
| Figure 3-48: Class 2 Device Recall Spacelabs Healthcare Xhibit Telemetry Receiver, Model 96280, |
| software version 1.1 79 |
| Figure 3-49: Class 2 Device Recall ELI 10, ELI 350, ELI 150c, ELI 250c, ELI 230, ELI 280, 80 |
| Figure 3-50: Class 2 Device Recall ELI PC 80 |
| Figure 3-51: Class 2 Device Recall 81 |
| Figure 3-52: Class 2 Device Recall Welch Allyn ProBP 2400 Digital Blood Pressure Device 81 |
| Figure 2-53: Wrist-based Blood Pressure Monitoring Study 82 |
| Figure 2-54: A Pilot Study of Hypertension Management Using Remote Patient Monitoring 82 |
| Figure 2-55: Hypertension Control and Remote Blood Pressure Monitoring 83 |
| Figure 3-56: Pediatric Cardiac Output Monitoring Observational Study (POGO) 84 |
| Figure 3-57: ClearSight System CHN Study 84 |
| Figure 3-58: Wearable Sensor for Responsive DBS for ET 85 |
| Figure 3-59: Novii External Fetal Monitoring Device (Novii) 86 |
| Figure 3-60: U-TruSignal SpO2 Testing in Neonates 86 |
| Figure 3-61: Transcutaneous Carbon Dioxide Monitoring in Neonates Receiving Therapeutic Hypothermia |
| for Neonatal Encephalopathy 87 |
| Figure 3-62: Validation of Non-Invasive Intracranial Pressure (ICP) Monitoring Device 88 |
| Figure 2-63: Feasibility and Performance Evaluation of INVSENSOR00024 89 |
| Figure 2-64: Feasibility and Performance Evaluation for INVSENSOR00024 89 |
| Figure 3-65: SpO2 Validation of Noninvasive Red Diamond Disposable Pulse Oximeter Sensor 90 |
| Figure 3-66: Accuracy of Oxygen Saturation (SpO2) Noninvasive Pulse Oximeter Sensor (RD Disposable) |
| Under Motion Conditions 90 |
| Figure 2-67: SpO2 Accuracy Validation of the OxySoft Sensor Via Reference CO-Oximetry Motion Study |
| 91 |
| Figure 2-68: Validation of Next Generation Cerebral and Tissue Oximeter 91 |
| Figure 3-69: BiOSENCY BORA Band SpO2 Validation Study 92 |
| Figure 2-70: Validation Study in Patients of a Non-invasive Blood Pressure Monitor for Perioperative Use |
| 93 |
| Figure 2-71: U-TruSignal SpO2 Testing in Neonates 94 |
| Figure 2-72: Longitudinal Coverage With Evidence Development Study on Micra AV Leadless Pacemakers |
| (Micra AV CED) 95 |
| Figure 2-73: Extravascular ICD Pivotal Study (EV ICD) 95 |
| Figure 2-74: Extravascular ICD Pilot Study (EV ICD) 96 |
| Figure 2-75: Benefits of ICD for the Primary Prevention in Patients With Valvular Cardiomyopathy (BEAT) |
| 96 |
| Figure 2-76: Longitudinal Coverage With Evidence Development Study on Micra Leadless Pacemakers |
| (Micra CED) 97 |
| Figure 2-77: Comparing the Accuracy of Different Blood Glucose Monitoring Systems (CGM and FGM) 97 |
| Figure 2-78: Clinical Evaluation of LifeScan Blood Glucose Monitoring Systems 98 |
| Figure 2-79: Evaluation of CIED "Readers" for Disease Management 98 |
| Figure 4-1: Patient Monitoring Equipment Device Market by Segment, Global, 2018 – 2028 (US$M) (1 of |
| 2) 101 |
| Figure 4-2: Patient Monitoring Equipment Device Market by Segment, Global, 2018 – 2028 (US$M) (2 of |
| 2) 102 |
| Figure 4-3: Patient Monitoring Equipment Device Market by Region, Global, 2018 – 2028 (US$M) 105 |
| Figure 4-4: Leading Competitors, Patient Monitoring Equipment Device Market by Segment, Global, 2021 |
| (1 of 2) 115 |
| Figure 4-5: Leading Competitors, Patient Monitoring Equipment Device Market by Segment, Global, 2021 |
| (2 of 2) 116 |
| Figure 4-6: List of Other Competitors, Patient Monitoring Equipment Market, Global, 2021 (1 of 2) 117 |
| Figure 4-7: List of Other Competitors, Patient Monitoring Equipment Market, Global, 2021 (2 of 2) 118 |
| Figure 4-8: SWOT Analysis, Edwards Lifesciences 137 |
| Figure 4-9: SWOT Analysis, GE Healthcare 138 |
| Figure 4-10: SWOT Analysis, Honeywell Life Sciences 139 |
| Figure 4-11: SWOT Analysis, Masimo 140 |
| Figure 4-12: SWOT Analysis, Medtronic 141 |
| Figure 4-13: SWOT Analysis, Mindray Medical 142 |
| Figure 4-14: SWOT Analysis, Natus Medical 143 |
| Figure 4-15: SWOT Analysis, Nihon Kohden 144 |
| Figure 4-16: SWOT Analysis, Omron Healthcare 145 |
| Figure 4-17: SWOT Analysis, Philips Healthcare 146 |
| Figure 4-18: SWOT Analysis, Spacelabs Healthcare 147 |
| Figure 4-19: SWOT Analysis, Welch Allyn 148 |
| Figure 5-1: Multi-Parameter Vital Signs Monitoring Markets Covered 153 |
| Figure 5-2: Multi-Parameter Vital Signs Monitoring Regions Covered, Global (1 of 2) 154 |
| Figure 5-3: Multi-Parameter Vital Signs Monitoring Regions Covered, Global (2 of 2) 155 |
| Figure 5-4: Multi-Parameter Vital Signs Monitoring Device Market by Segment, Global, 2018 – 2028 |
| (US$M) 160 |
| Figure 5-5: Multi-Parameter Vital Signs Monitoring Device Market by Region, Global, 2018 – 2028 |
| (US$M) 162 |
| Figure 5-6: Low-Acuity Vital Signs Monitoring Market, Global, 2018 – 2028 165 |
| Figure 5-7: Units Sold by Region, Low-Acuity Vital Signs Monitoring Market, Global, 2018 – 2028 166 |
| Figure 5-8: Average Selling Price by Region, Low-Acuity Vital Signs Monitoring Market, Global, 2018 – |
| 2028 (US$) 167 |
| Figure 5-9: Market Value by Region, Low-Acuity Vital Signs Monitoring Market, Global, 2018 – 2028 |
| (US$M) 168 |
| Figure 5-10: Mid-Acuity Vital Signs Monitoring Market, Global, 2018 – 2028 170 |
| Figure 5-11: Units Sold by Region, Mid-Acuity Vital Signs Monitoring Market, Global, 2018 – 2028 171 |
| Figure 5-12: Average Selling Price by Region, Mid-Acuity Vital Signs Monitoring Market, Global, 2018 – |
| 2028 (US$) 172 |
| Figure 5-13: Market Value by Region, Mid-Acuity Vital Signs Monitoring Market, Global, 2018 – 2028 |
| (US$M) 173 |
| Figure 5-14: High-Acuity Vital Signs Monitoring Market, Global, 2018 – 2028 174 |
| Figure 5-15: Units Sold by Region, High-Acuity Vital Signs Monitoring Market, Global, 2018 – 2028 175 |
| Figure 5-16: Average Selling Price by Region, High-Acuity Vital Signs Monitoring Market, Global, 2018 – |
| 2028 (US$) 176 |
| Figure 5-17: Market Value by Region, High-Acuity Vital Signs Monitoring Market, Global, 2018 – 2028 |
| (US$M) 177 |
| Figure 5-18: Central Station Monitoring Market, Global, 2018 – 2028 179 |
| Figure 5-19: Units Sold by Region, Central Station Monitoring Market, Global, 2018 – 2028 180 |
| Figure 5-20: Average Selling Price by Region, Central Station Monitoring Market, Global, 2018 – 2028 |
| (US$) 181 |
| Figure 5-21: Market Value by Region, Central Station Monitoring Market, Global, 2018 – 2028 (US$M)182 |
| Figure 5-22: Leading Competitors, Multi-Parameter Vital Signs Monitoring Market, Global, 2021 191 |
| Figure 5-23: Leading Competitors, Multi-Parameter Vital Signs Monitoring Market, Global, 2021 192 |
| Figure 6-1: Wireless Ambulatory Telemetry Monitoring Markets Covered 198 |
| Figure 6-2: Wireless Ambulatory Telemetry Monitoring Regions Covered, Global (1 of 2) 199 |
| Figure 6-3: Wireless Ambulatory Telemetry Monitoring Regions Covered, Global (2 of 2) 200 |
| Figure 6-4: Wireless Ambulatory Telemetry Monitoring Device Market by Segment, Global, 2018 – 2028 |
| (US$M) 204 |
| Figure 6-5: Wireless Ambulatory Telemetry Monitoring Device Market by Region, Global, 2018 – 2028 |
| (US$M) 206 |
| Figure 6-6: Patient-Worn Monitor Market, Global, 2018 – 2028 208 |
| Figure 6-7: Units Sold by Region, Patient-Worn Monitor Market, Global, 2018 – 2028 209 |
| Figure 6-8: Average Selling Price by Region, Patient-Worn Monitor Market, Global, 2018 – 2028 (US$) 210 |
| Figure 6-9: Market Value by Region, Patient-Worn Monitor Market, Global, 2018 – 2028 (US$M) 211 |
| Figure 6-10: Wireless Ambulatory Telemetry Central Station Market, Global, 2018 – 2028 212 |
| Figure 6-11: Units Sold by Region, Wireless Ambulatory Telemetry Central Station Market, Global, 2018 – 2028 213 |
| Figure 6-12: Average Selling Price by Region, Wireless Ambulatory Telemetry Central Station Market, |
| Global, 2018 – 2028 (US$) 214 |
| Figure 6-13: Market Value by Region, Wireless Ambulatory Telemetry Central Station Market, Global, |
| 2018 – 2028 (US$M) 215 |
| Figure 6-14: Leading Competitors, Wireless Ambulatory Telemetry Monitoring Market, Global, 2021 .. 223 |
| Figure 7-1: Electromyogram Monitoring Markets Covered 229 |
| Figure 7-2: Electromyogram Monitoring Regions Covered, Global (1 of 2) 230 |
| LIST OF FIGURES |
| © iData Research Inc., 2022 xvii |
| Figure 7-3: Electromyogram Monitoring Regions Covered, Global (2 of 2) 231 |
| Figure 7-4: Electromyogram Monitoring Market by Segment, Global, 2018 – 2028 (US$M) 235 |
| Figure 7-5: Electromyogram Monitoring Device Market by Region, Global, 2018 – 2028 (US$M) 237 |
| Figure 7-6: EMG Monitor Market, Global, 2018 – 2028 239 |
| Figure 7-7: Units Sold by Region, EMG Monitor Market, Global, 2018 – 2028 240 |
| Figure 7-8: Average Selling Price by Region, EMG Monitor Market, Global, 2018 – 2028 (US$) 241 |
| Figure 7-9: Market Value by Region, EMG Monitor Market, Global, 2018 – 2028 (US$M) 242 |
| Figure 7-10: EMG Consumables Market by Segment, Global, 2018 – 2028 (US$M) 243 |
| Figure 7-11: Total EMG Consumables Market, Global, 2018 – 2028 244 |
| Figure 7-12: Units Sold by Region, EMG Consumables Market, Global, 2018 – 2028 245 |
| Figure 7-13: Average Selling Price by Region, EMG Consumables Market, Global, 2018 – 2028 (US$) 246 |
| Figure 7-14: Market Value by Region, EMG Consumables Market, Global, 2018 – 2028 (US$M) 247 |
| Figure 7-15: EMG Concentric Needle Market, Global, 2018 – 2028 248 |
| Figure 7-16: Units Sold by Region, EMG Concentric Needle Market, Global, 2018 – 2028 249 |
| Figure 7-17: Average Selling Price by Region, EMG Concentric Needle Market, Global, 2018 – 2028 (US$) |
| 250 |
| Figure 7-18: Market Value by Region, EMG Concentric Needle Market, Global, 2018 – 2028 (US$M) 251 |
| Figure 7-19: EMG Monopolar Needle Market, Global, 2018 – 2028 252 |
| Figure 7-20: Units Sold by Region, EMG Monopolar Needle Market, Global, 2018 – 2028 253 |
| Figure 7-21: Average Selling Price by Region, EMG Monopolar Needle Market, Global, 2018 – 2028 (US$) |
| 254 |
| Figure 7-22: Market Value by Region, EMG Monopolar Needle Market, Global, 2018 – 2028 (US$M) 255 |
| Figure 7-23: EMG Surface Electrode Market, Global, 2018 – 2028 256 |
| Figure 7-24: Units Sold by Region, EMG Surface Electrode Market, Global, 2018 – 2028 257 |
| Figure 7-25: Average Selling Price by Region, EMG Surface Electrode Market, Global, 2018 – 2028 (US$) |
| 258 |
| Figure 7-26: Market Value by Region, EMG Surface Electrode Market, Global, 2018 – 2028 (US$M) 259 |
| Figure 7-27: Leading Competitors, Electromyogram Monitoring Market, Global, 2021 266 |
| Figure 8-1: Electroencephalogram Monitoring Markets Covered 272 |
| Figure 8-2: Electroencephalogram Monitoring Regions Covered, Global (1 of 2) 273 |
| Figure 8-3: Electroencephalogram Monitoring Regions Covered, Global (2 of 2) 274 |
| Figure 8-4: EEG Wave Indicators of Physiological State 277 |
| Figure 8-5: Electroencephalogram Monitoring Device Market by Segment, Global, 2018 – 2028 (US$M) |
| 280 |
| Figure 8-6: Electroencephalogram Monitoring Device Market by Region, Global, 2018 – 2028 (US$M) 282 |
| Figure 8-7: EEG Monitor Market, Global, 2018 – 2028 284 |
| Figure 8-8: Units Sold by Region, EEG Monitor Market, Global, 2018 – 2028 285 |
| Figure 8-9: Average Selling Price by Region, EEG Monitor Market, Global, 2018 – 2028 (US$) 286 |
| Figure 8-10: Market Value by Region, EEG Monitor Market, Global, 2018 – 2028 (US$M) 287 |
| Figure 8-11: EEG Electrode Market by Segment, Global, 2018 – 2028 (US$M) 289 |
| Figure 8-12: Total EEG Electrode Market, Global, 2018 – 2028 290 |
| Figure 8-13: Units Sold by Region, EEG Electrode Market, Global, 2018 – 2028 291 |
| Figure 8-14: Average Selling Price by Region, EEG Electrode Market, Global, 2018 – 2028 (US$) 292 |
| Figure 8-15: Market Value by Region, EEG Electrode Market, Global, 2018 – 2028 (US$M) 293 |
| Figure 8-16: Disposable EEG Electrode Market, Global, 2018 – 2028 294 |
| Figure 8-17: Units Sold by Region, Disposable EEG Electrode Market, Global, 2018 – 2028 295 |
| Figure 8-18: Average Selling Price by Region, Disposable EEG Electrode Market, Global, 2018 – 2028 |
| (US$) 296 |
| Figure 8-19: Market Value by Region, Disposable EEG Electrode Market, Global, 2018 – 2028 (US$M) 297 |
| Figure 8-20: Reusable EEG Electrode Market, Global, 2018 – 2028 298 |
| Figure 8-21: Units Sold by Region, Reusable EEG Electrode Market, Global, 2018 – 2028 299 |
| Figure 8-22: Average Selling Price by Region, Reusable EEG Electrode Market, Global, 2018 – 2028 (US$) |
| 300 |
| Figure 8-23: Market Value by Region, Reusable EEG Electrode Market, Global, 2018 – 2028 (US$M) 301 |
| Figure 8-24: Leading Competitors, Electroencephalogram Monitoring Market, Global, 2021 309 |
| Figure 9-1: Fetal and Neonatal Monitoring Markets Covered 315 |
| Figure 9-2: Fetal and Neonatal Monitoring Regions Covered, Global (1 of 2) 316 |
| Figure 9-3: Fetal and Neonatal Monitoring Regions Covered, Global (2 of 2) 317 |
| Figure 9-4: Fetal and Neonatal Monitoring Device Market by Segment, Global, 2018 – 2028 (US$M) 321 |
| Figure 9-5: Fetal and Neonatal Monitoring Device Market by Region, Global, 2018 – 2028 (US$M) 323 |
| Figure 9-6: Fetal Monitoring Market, Global, 2018 – 2028 325 |
| Figure 9-7: Units Sold by Region, Fetal Monitoring Market, Global, 2018 – 2028 326 |
| Figure 9-8: Average Selling Price by Region, Fetal Monitoring Market, Global, 2018 – 2028 (US$) 327 |
| Figure 9-9: Market Value by Region, Fetal Monitoring Market, Global, 2018 – 2028 (US$M) 328 |
| Figure 9-10: Neonatal Monitoring Market, Global, 2018 – 2028 329 |
| Figure 9-11: Units Sold by Region, Neonatal Monitoring Market, Global, 2018 – 2028 330 |
| Figure 9-12: Average Selling Price by Region, Neonatal Monitoring Market, Global, 2018 – 2028 (US$) 331 |
| Figure 9-13: Market Value by Region, Neonatal Monitoring Market, Global, 2018 – 2028 (US$M) 332 |
| Figure 9-14: Leading Competitors, Fetal and Neonatal Monitoring Market, Global, 2021 339 |
| Figure 10-1: Pulse Oximetry Monitoring Markets Covered 345 |
| Figure 10-2: Pulse Oximetry Monitoring Regions Covered, Global (1 of 2) 346 |
| Figure 10-3: Pulse Oximetry Monitoring Regions Covered, Global (2 of 2) 347 |
| Figure 10-4: Pulse Oximetry Monitoring Device Market by Segment, Global, 2018 – 2028 (US$M) 351 |
| Figure 10-5: Pulse Oximetry Monitoring Device Market by Region, Global, 2018 – 2028 (US$M) 353 |
| Figure 10-6: Pulse Oximetry Monitor Market by Segment, Global, 2018 – 2028 (US$M) 356 |
| Figure 10-7: Bedside Pulse Oximetry Monitor Market, Global, 2018 – 2028 357 |
| Figure 10-8: Units Sold by Region, Bedside Pulse Oximetry Monitor Market, Global, 2018 – 2028 358 |
| Figure 10-9: Average Selling Price by Region Bedside Pulse Oximetry Monitor Market, Global, 2018 – |
| 2028 (US$) 359 |
| Figure 10-10: Market Value by Region, Bedside Pulse Oximetry Monitor Market, Global, 2018 – 2028 |
| (US$M) 360 |
| Figure 10-11: Handheld Pulse Oximetry Monitor Market, Global, 2018 – 2028 361 |
| Figure 10-12: Units Sold by Region, Handheld Pulse Oximetry Monitor Market, Global, 2018 – 2028 362 |
| Figure 10-13: Average Selling Price by Region, Handheld Pulse Oximetry Monitor Market, Global, 2018 – |
| 2028 (US$) 363 |
| Figure 10-14: Market Value by Region, Handheld Pulse Oximetry Monitor Market, Global, 2018 – 2028 |
| (US$M) 364 |
| Figure 10-15: Pulse Oximetry Monitoring Sensor Market by Segment, Global, 2018 – 2028 (US$M) 366 |
| Figure 10-16: Disposable Pulse Oximetry Monitoring Sensor Market, Global, 2018 – 2028 367 |
| Figure 10-17: Units Sold by Region, Disposable Pulse Oximetry Monitoring Sensor Market, Global, 2018 – |
| 2028 368 |
| Figure 10-18: Average Selling Price by Region, Disposable Pulse Oximetry Monitoring Sensor Market, |
| Global, 2018 – 2028 (US$) 369 |
| Figure 10-19: Market Value by Region, Disposable Pulse Oximetry Monitoring Sensor Market, Global, |
| 2018 – 2028 (US$M) 370 |
| Figure 10-20: Reusable Pulse Oximetry Monitoring Sensor Market, Global, 2018 – 2028 371 |
| Figure 10-21: Units Sold by Region, Reusable Pulse Oximetry Monitoring Sensor Market, Global, 2018 – |
| 2028 372 |
| Figure 10-22: Average Selling Price by Region, Reusable Pulse Oximetry Monitoring Sensor Market, |
| Global, 2018 – 2028 (US$) 373 |
| Figure 10-23: Market Value by Region, Reusable Pulse Oximetry Monitoring Sensor Market, Global, 2018 |
| – 2028 (US$M) 374 |
| Figure 10-24: Leading Competitors, Pulse Oximetry Monitoring Market, Global, 2021 380 |
| Figure 11-1: Blood Pressure Monitoring Markets Covered 386 |
| Figure 11-2: Blood Pressure Monitoring Regions Covered, Global (1 of 2) 387 |
| Figure 11-3: Blood Pressure Monitoring Regions Covered, Global (2 of 2) 388 |
| Figure 11-4: Blood Pressure Monitoring Device Market by Segment, Global, 2018 – 2028 (US$M) 391 |
| Figure 11-5: Blood Pressure Monitoring Device Market by Region, Global, 2018 – 2028 (US$M) 393 |
| Figure 11-6: Personal Blood Pressure Monitoring Market, Global, 2018 – 2028 395 |
| Figure 11-7: Units Sold by Region, Personal Blood Pressure Monitoring Market, Global, 2018 – 2028 396 |
| Figure 11-8: Average Selling Price by Region, Personal Blood Pressure Monitoring Market, Global, 2018 – |
| 2028 (US$) 397 |
| Figure 11-9: Market Value by Region, Personal Blood Pressure Monitoring Market, Global, 2018 – 2028 |
| (US$M) 398 |
| Figure 11-10: Professional Blood Pressure Monitoring Market, Global, 2018 – 2028 399 |
| Figure 11-11: Units Sold by Region, Professional Blood Pressure Monitoring Market, Global, 2018 – 2028 |
| 400 |
| Figure 11-12: Average Selling Price by Region, Professional Blood Pressure Monitoring Market, Global, |
| 2018 – 2028 (US$) 401 |
| Figure 11-13: Market Value by Region, Professional Blood Pressure Monitoring Market, Global, 2018 – |
| 2028 (US$M) 402 |
| Figure 11-14: Leading Competitors, Blood Pressure Monitoring Market, Global, 2021 408 |
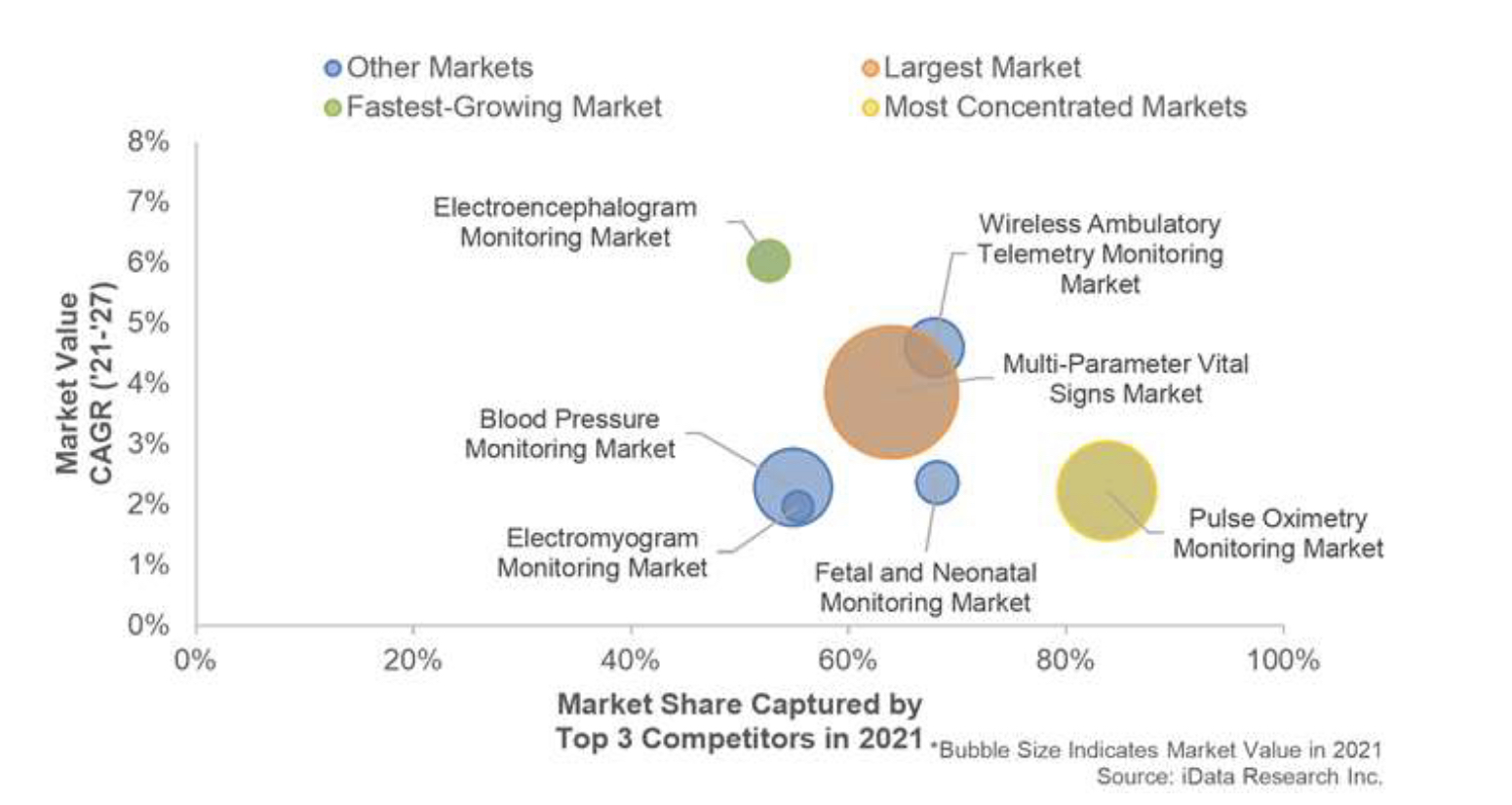 Globally, there is an increased awareness among both physicians and patients as to the benefits of monitoring in a wide array of areas, including continual monitoring both in hospital as well as in the home. This is not only driving demand for existing devices, but also motivating innovation in the market across multiple segments. As devices continue to improve their array of features and lower their costs, the healthcare population will be more motivated to act on this awareness to purchase new devices.
Globally, there is an increased awareness among both physicians and patients as to the benefits of monitoring in a wide array of areas, including continual monitoring both in hospital as well as in the home. This is not only driving demand for existing devices, but also motivating innovation in the market across multiple segments. As devices continue to improve their array of features and lower their costs, the healthcare population will be more motivated to act on this awareness to purchase new devices. Philips Healthcare was the leading competitor in the global patient monitoring equipment market. Philips led a number of notable segments, including multi-parameter vital signs monitoring, wireless ambulatory telemetry monitoring and fetal and neonatal monitoring. Philips has a globally recognized brand that it is able to leverage into a strong market position in multiple regional markets.
Philips Healthcare was the leading competitor in the global patient monitoring equipment market. Philips led a number of notable segments, including multi-parameter vital signs monitoring, wireless ambulatory telemetry monitoring and fetal and neonatal monitoring. Philips has a globally recognized brand that it is able to leverage into a strong market position in multiple regional markets.





