Product Description
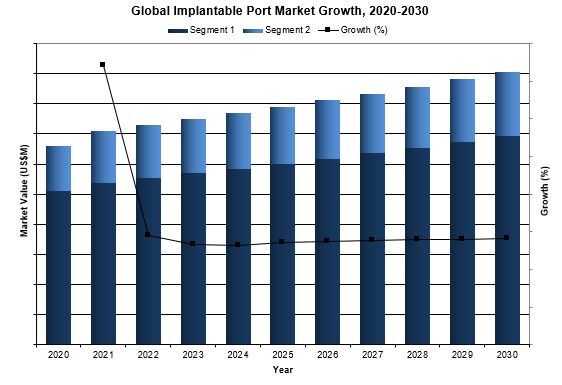
Overall, the global implantable port market was valued slightly over $370 million in 2023. This is expected to increase over the forecast period at a CAGR of 2.8% to reach about $450 million.
Throughout this medical market research, we analyzed over 15 implantable port companies across 7 continents and used our comprehensive methodology to understand the market sizes, unit sales, company market shares, and to create accurate forecasts. The full report suite on the global market for implantable ports includes non-power-injectable and power-injectable implantable ports.
DATA TYPES INCLUDED
- Unit Sales, Average Selling Prices, Market Value & Growth Trends
- Forecasts Until 2030, and Historical Data to 2020
- Market Drivers & Limiters for Each Segment
- Competitive Analysis with Market Shares for Each Segment
- Recent Mergers & Acquisitions
- COVID19 Impact
- Disease Overviews and Demographic Information
- Company Profiles, Product Portfolios and SWOT for Top Competitors
Global Implantable Port Market Insights
New technologies are expected to boost the implantable port market, with a growing interest in peripheral placement for site-specific cancer treatment. Although implantable ports exhibit the lowest infection rate among vascular access devices, timely diagnosis and patient immunity remain crucial factors. Ongoing and future advancements are prioritizing reducing infection rates by applying antimicrobial coatings to catheters, enhancing the appeal of ports and fostering competition with PICCs and CVCs.
Global Implantable Port Market Share Insights

- In 2023, Becton Dickinson led the implantable port market with the versatile PowerPort® line, including options like the titanium PowerPort® Slim and the lightweight PowerPort® MRI®. They also offered non-power-injectable alternatives like the SlimPort™ Dual-Lumen Rosenblatt™ and the X-Port™.
- In 2023, AngioDynamics made headway in the implantable port market with innovative products such as Smart Port CT™ featuring integrated Vortex® technology. While the BioFlo® technology succeeded in preventing thrombus formation, the discontinuation of Morpheus® PICC had a more significant impact on PICC sales.
- In 2023, ICU Medical held the third spot among implantable port market competitors. They were known for the PORT-A-CATH® brand, particularly the MRI-safe PORT-A-CATH II® model. Additionally, they provided hybrid P. A. S. PORT® arm series options. However, the adoption of arm ports was limited due to concerns about catheter pinching due to patient movement.
Market Segmentation Summary
- Non-Power-Injectable Implantable Port Market
- Power-Injectable Implantable Port Market
Global Research Scope Summary
| Report Attribute | Details |
|---|---|
| Regions | North America (Canada, United States) Latin America (Argentina, Brazil, Chile, Colombia, Mexico, Peru, Venezuela) Western Europe (Austria, Benelux, France, Germany, Italy, Portugal, Scandinavia, Spain, Switzerland, U.K.) Central & Eastern Europe (Baltic States, Bulgaria, Croatia, Czech Republic, Greece, Hungary, Kazakhstan, Poland, Romania, Russia, Turkey, Ukraine) Middle East (Bahrain, Iran, Israel, Kuwait, Oman, Qatar, Saudi Arabia, United Arab Emirates) Asia Pacific (Australia, Cambodia, China, Hong Kong, India, Indonesia, Japan, Malaysia, Myanmar, New Zealand, Philippines, Singapore, South Korea, Taiwan, Thailand, Vietnam) Africa (Algeria, Egypt, Ghana, Kenya, Libya, Morocco, Nigeria, South Africa, Sudan, Uganda) |
| Base Year | 2023 |
| Forecast | 2024-2030 |
| Historical Data | 2020-2023 |
| Quantitative Coverage | Market Size, Market Shares, Market Forecasts, Market Growth Rates, Units Sold, and Average Selling Prices. |
| Qualitative Coverage | COVID19 Impact, Market Growth Trends, Market Limiters, Competitive Analysis & SWOT for Top Competitors, Mergers & Acquisitions, Company Profiles, Product Portfolios, FDA Recalls, Disruptive Technologies, Disease Overviews. |
| Data Sources | Primary Interviews with Industry Leaders, Government Physician Data, Regulatory Data, Hospital Private Data, Import & Export Data, iData Research Internal Database. |
CONTACT US FOR ADDITIONAL INFORMATION
For full segmentation and any questions regarding research coverage, please contact us for a complimentary demo of the full report.






