Product Description
Overall, the global bronchoscopic biopsy device market was valued at $134.8 million in 2022. This is expected to increase over the forecast period at a CAGR of 9.0% to reach $247.1 million.
The full report suite on the global market for bronchoscopic biopsy devices includes segments of bronchial forceps and transbronchial needle aspiration (TBNA) needle devices.
MARKET DATA INCLUDED
- Unit Sales, Average Selling Prices, Market Size & Growth Trends
- Procedure Numbers
- COVID-19 Impact Analysis
- Market Drivers & Limiters
- Market Forecasts Until 2029, and Historical Data to 2019
- Recent Mergers & Acquisitions
- Company Profiles and Product Portfolios
- Leading Competitors
GLOBAL BRONCHOSCOPIC BIOPSY DEVICE MARKET INSIGHTS
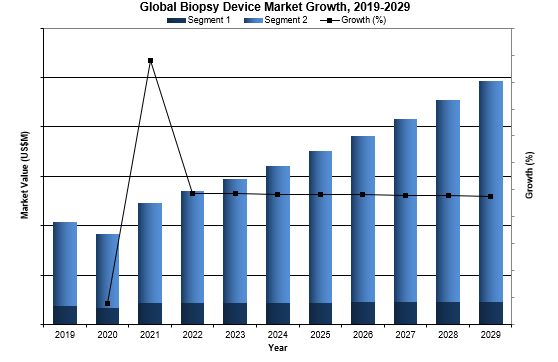 One major market driver is innovation in the TBNA market. The newly developed products are on the higher end of the price range that will drive up the market growth.
One major market driver is innovation in the TBNA market. The newly developed products are on the higher end of the price range that will drive up the market growth.
GLOBAL BRONCHOSCOPIC BIOPSY DEVICE MARKET SHARE INSIGHTS
The Global bronchoscopic biopsy device market was controlled by Olympus, Boston Scientific, and Cook Medical. Olympus was the leading competitor in the bronchoscopic biopsy device market in 2022, with a majority of the market share. It participated in the disposable bronchial forceps and both TBNA markets. The company was the leading competitor in the endobronchial ultrasound (EBUS)-TBNA market, with a majority of the market share. The company offers an expanded line of TBNA needles under the ViziShot® product line, which has had a market presence for over a decade.
Olympus was the leading competitor in the bronchoscopic biopsy device market in 2022, with a majority of the market share. It participated in the disposable bronchial forceps and both TBNA markets. The company was the leading competitor in the endobronchial ultrasound (EBUS)-TBNA market, with a majority of the market share. The company offers an expanded line of TBNA needles under the ViziShot® product line, which has had a market presence for over a decade.
MARKET SEGMENTATION SUMMARY
- Bronchoscopic Biopsy Device Market – MedCore – Includes:
- Segments of bronchial forceps and transbronchial needle aspiration (TBNA) needle devices.
Research Scope Summary
| Report Attribute | Details |
|---|---|
| Regions | North America (Canada, United States) Latin America (Argentina, Brazil, Chile, Colombia, Mexico, Peru, Venezuela) Western Europe (Austria, Benelux, France, Germany, Italy, Portugal, Scandinavia, Spain, Switzerland, U.K.) Central & Eastern Europe (Baltic States, Bulgaria, Croatia, Czech Republic, Greece, Hungary, Kazakhstan, Poland, Romania, Russia, Turkey, Ukraine) Middle East (Bahrain, Iran, Israel, Kuwait, Oman, Qatar, Saudi Arabia, United Arab Emirates) Asia Pacific (Australia, Cambodia, China, Hong Kong, India, Indonesia, Japan, Malaysia, Myanmar, New Zealand, Philippines, Singapore, South Korea, Taiwan, Thailand, Vietnam) Africa (Algeria, Egypt, Ghana, Kenya, Libya, Morocco, Nigeria, South Africa, Sudan, Uganda) |
| Base Year | 2022 |
| Forecast Period | 2023-2029 |
| Historical Data | 2019-2022 |
| Quantitative Data | Market Size, Market Share, Market Growth Rates, Units Sold, Average Selling Prices |
| Qualitative Data | COVID-19 Impact, Market Growth Trends, Market Limiters, Competitive Analysis & SWOT for Top Competitors, Mergers & Acquisitions, Company Profiles, Product Portfolios |
| Data Sources | Primary Interviews with Industry Leaders, Government Physician Data, Regulatory Data, Hospital Private Data, Import & Export Data.
Read more about iData’s 9-Step Research Methodology |
WHY CHOOSE IDATA?
- Experts Only – iData has been around for over 17 years and is the trusted source of intelligence, and data-driven insights for key industry players. We are made up of specialists with a sole focus on medical devices, dental devices and pharmaceuticals. Our research and consulting field of endeavour never strays from the medical device industry and, because of this, we are the number one choice for leading companies in this field.
- Global Coverage – iData’s unique methodology, and its expansion to over 70 countries world-wide, has made it one of the most viable sources of intelligence for global companies along with those who plan to expand their portfolio beyond the confines of their own country. Providing procedural data at the country level is another testimony to the trusted global coverage we provide.
- Attention to Detail – It’s our attention to small details, scheduling of timelines, and keen project management that makes us stand out from the rest. We are creative, and our reports include metrics such as procedure numbers, ASPs, and SKU-level insights that are not found elsewhere.
- Pricing – When comparing like-with-like, iData’s prices are not only extremely competitive but also the most cost-effective. We strive for success and want the same for our clients. With the level of detail incorporated into each report alongside the extensive segmentation provided, no other report compares at our price point.






